SAVE 17% OFF 17% OFF Use Code LUCKY17 *

Mirataz for Cats - Transdermal Ointment (5 gm) - [Weight Loss Management]
- Description
- Directions
- Reviews
Description
Mirataz™ is indicated for the management of weight loss in cats. Mirataz™ (mirtazapine transdermal ointment) is a white to off-white ointment containing 2% (w/w) of mirtazapine suitable for transdermal (topical) administration.
- Mirataz is the first and only FDA-approved transdermal medication for the management of weight loss in cats
- In clinical studies, Mirataz resulted in significant weight gain in cats in as little as 14 days following topical application of 2 mg oer day8
- Mirataz gives your clients a practical way to manage their cat's weight loss without administration of oral medication and does not rely on the cat to eat to be medicated
- Due to proprietary Accusorb™ technology, Mirataz achieves measurable plasma concentrations of mirtazapine in cats9
- Mirataz was well tolerated both locally and systemically in clinical studies8
Mirataz™ is contraindicated in cats with a known hypersensitivity to mirtazapine or to any of the excipients.
Directions
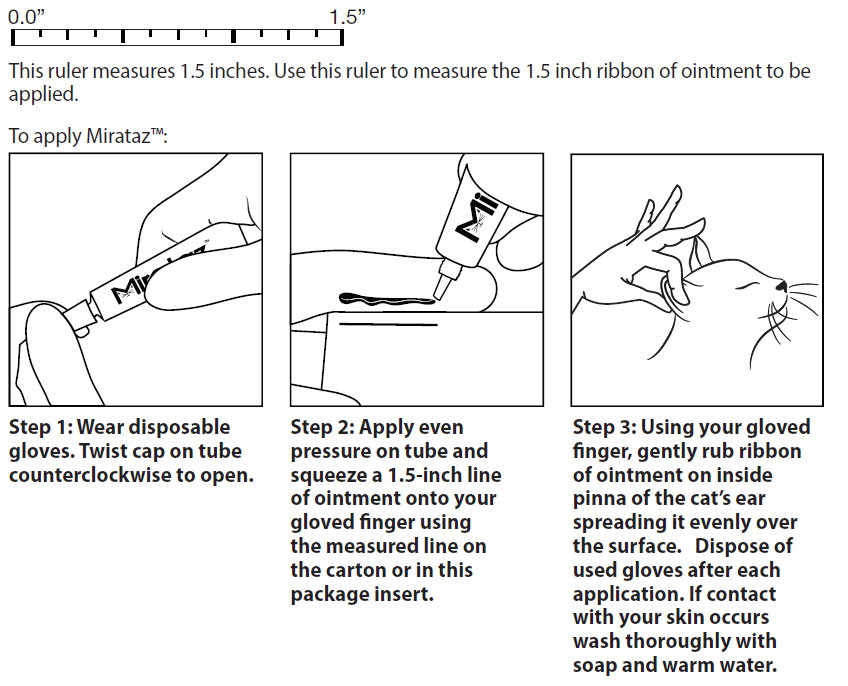
Administer topically by applying a 1.5-inch ribbon of ointment (approximately 2 mg/cat) on the inner pinna of the cat's ear once daily for 14 days (see Diagrams below).
Wear disposable gloves when applying Mirataz™ Dispose of used gloves after each application.
Alternate the daily application of Mirataz™ between the left and right inner pinna of the ears. Do not administer into the external ear canal. If desired, the inner pinna of the cat's ear may be cleaned by wiping with a dry tissue or cloth immediately before the next scheduled dose. If a dose is missed, apply Mirataz™ the following day and resume daily dosing.
View Mirataz Drug Facts Sheet.
View Mirataz Safety Data Sheet (MSDS).
To demonstrate the method of administering the dose, the veterinarian or trained personnel at the clinic should apply the first dose in the presence of the owner.
Contraindications
Mirataz™ is contraindicated in cats with a known hypersensitivity to mirtazapine or to any of the excipients.Mirataz™ should not be given in combination, or within 14 days before or after treatment with a monoamine oxidase inhibitor (MAOI) [e.g., selegiline hydrochloride (L-deprenyl), amitraz], as there may be an increased risk of serotonin syndrome.
Human Warnings
Not for human use. Keep out of reach of children.Wear disposable gloves when handling or applying Mirataz™ to prevent accidental topical exposure. After application disposes of used gloves and washes hands with soap and water. After application, care should be taken that people or other animals in the household do not come in contact with the treated cat for 2 hours because mirtazapine can be absorbed transdermally and orally. However, negligible residues are present at the application site and the body of the cat at 2 hours after dosing.
In case of accidental skin exposure, wash thoroughly with soap and warm water. In case of accidental eye exposure, flush eyes with water. If skin or eye irritation occurs seek medical attention.
In case of accidental ingestion, or if skin or eye irritation occurs, seek medical attention.
Precautions: Do not administer orally or to the eye.
Use with caution in cats with hepatic disease. Mirtazapine may cause elevated serum liver enzymes (See Animal Safety).
Use with caution in cats with kidney disease. Kidney disease may cause clearance of mirtazapine which may result in higher drug exposure.
Upon discontinuation of Mirataz™ it is important to monitor the cat's food intake. Food intake may lessen after discontinuation of mirtazapine transdermal ointment. If food intake diminishes dramatically (>75%) for several days, or if the cat stops eating for more than 48 hours, reevaluate the cat.
Mirataz™ has not been evaluated in cats < 2 kg or less than six months of age. The safe use of Mirataz™ has not been evaluated in cats that are intended for breeding, pregnant, or lactating cats.
ANIMAL SAFETY: The margin of safety of mirtazapine was evaluated in one laboratory study, a comprehensive review of six pilot studies (five laboratories and one clinical) utilizing the final market formulation, and one laboratory study that was not final market formulation.
Reviews
- Works within hours of application. Effects last (for my cat) 5 days before the need to reapply. Easier to administer than pills.
- Application is inner ear leather.
- Loud vocalization, just need to remind her to go eat!
- Easy to apply
- Works great
- Cat hated taking pills which is the alternative
- No biting or scratching while applying. Cat likes the ear scratch!
- None. Love this product and so does Sheba.


