SAVE 20% OFF 20% OFF Use Code PREZ20 *
Bravecto Topical Solution for Cats 6.3-13.8 lbs (4 tubes)|12 Months Protection - [Flea & Tick]
- Notice
- Description
- Ingredients
- Directions
- FAQ
- Reviews
Notices
This is our Rock-Bottom-Price! Sorry, Promotional offers do not apply (See Exclusions.)
Description
Bravecto for Cats is a powerful, vet-recommended topical flea and tick treatment that delivers up to 12 weeks of continuous protection with a single dose. Specially formulated for cats and kittens 6 months of age and older (weighing at least 2.6 lbs), Bravecto helps protect your pet from harmful pests with fewer applications per year—making it a convenient, worry-free choice for pet parents.
Bravecto kills 100% of fleas within 8 hours of application and effectively breaks the entire flea life cycle, from egg to adult. It also protects against black-legged ticks (Ixodes scapularis) for 12 weeks and controls Dermacentor variabilis (American dog tick) for 8 weeks. Its long-lasting formula means fewer missed doses, consistent protection, and less stress for both you and your cat.
Key Benefits
- Kills fleas fast—100% eliminated within 8 hours
- Up to 12 weeks of protection against fleas and black-legged ticks
- Treats and controls American dog tick (Dermacentor variabilis) for 8 weeks
- Breaks the 3-month flea life cycle with a single application
- Only 4 treatments per year provide year-round protection
- Fewer doses reduce the chance of missed treatments and gaps in coverage
- Easy, mess-free topical application
- Prescription-strength formula from trusted veterinary brand
Animals Treated
Approved for cats and kittens 6 months and older, weighing 2.6 lbs or more.
How It Works
Bravecto contains Fluralaner, an advanced insecticide from the isoxazoline class. After topical application to the skin, Fluralaner is absorbed into your cat’s bloodstream and distributed throughout the body. When fleas or ticks bite, they ingest the active ingredient, which disrupts their nervous system and causes rapid death.
This systemic mode of action ensures fast and effective protection that lasts for up to 12 weeks. Fluralaner is safe for long-term use and is trusted by veterinarians to control flea and tick infestations with fewer treatments.
Indications
Bravecto Topical Solution for Cats is indicated for the treatment and prevention of flea (Ctenocephalides felis) and tick (Ixodes scapularis and Dermacentor variabilis) infestations in cats 6 months of age and older, weighing 2.6 lbs or more.
Apply directly to the skin at the base of the cat’s neck as directed by your veterinarian. For best results, follow dosing guidelines closely and use year-round for consistent protection.
Bravecto is a registered trademark of Intervet Inc., a subsidiary of Merck Animal Health.
Specifications
- Brand: Bravecto
- Type: Topical flea and tick solution
- Indicated for: Flea and tick prevention in cats
- Active Ingredient: Fluralaner
- Form: Topical application (pipette)
- Application Site: Skin at the back of the neck
- Duration of Protection: Up to 12 weeks against fleas and black-legged ticks; 8 weeks for American dog ticks
- Age/Weight Requirements: 6 months or older; at least 2.6 lbs
- Safety: Approved for long-term use under veterinary guidance
- Storage: Store in original packaging at room temperature; avoid heat and moisture
- Manufacturer: Merck Animal Health
- Prescription Required: Yes
Ingredients
| Bravecto for Cats 2.6-6.2 lbs | |
|---|---|
| Active Ingredient (per dose) | Amount |
| Fluralaner | 112.5 mg |
| Bravecto for Cats 6.2-13.8 lbs | |
| Active Ingredients (per dose) | Amount |
| Fluralaner | 250 mg |
| Bravecto for Cats 13.8- 27.5 lbs | |
| Active Ingredients (per dose) | Amount |
| Fluralaner | 500 mg |
Directions
View Bravecto Topical Solution Drug Facts Sheet.
Dosage
Bravecto should be administered topically as a single dose every 12 weeks according to the Dosage Schedule below to provide a minimum dose of 18.2 mg/lb (40 mg/kg) body weight.
Bravecto may be administered every 8 weeks in case of potential exposure to Dermacentor variabilisticks (see Effectiveness).
| Dosage Schedule | ||
|---|---|---|
| Body Weight Ranges (lb) | Fluralaner content (mg/tube) | Tubes Administered |
| 2.6 - 6.2 | 112.5 | 1 (Green) |
| >6.2 - 13.8 | 250 | 1 (Blue) |
| >13.8 - 27.5* | 500 | 1 (Purple) |
*Cats over 27.5 lb should be administered the appropriate combination of tubes.
Administration
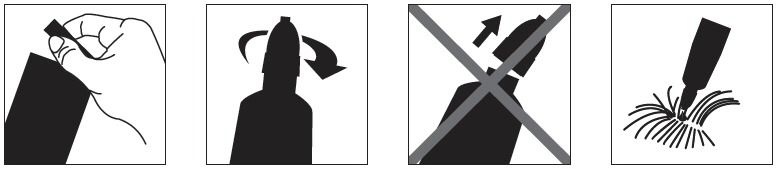
- Immediately before use, open the pouch and remove the tube. Hold thetube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counterclockwise one full turn. The cap is designed to stay on the tube for dosing and should not be removed. The tube is openand ready for application when a "click" is heard or felt. This indicates that the seal is broken and product can beapplied to the cat.
- The cat should be standing or lying with its back horizontol duringapplication. Part the fur at the administration site. Place the tube tip vertically against the skin at the base of theskull of the cat.
- Squeeze the tube and gently apply the entire contents of Bravectodirectly to the skin at the base of the skull of the cat. Avoid applying an excessive amount of solution that couldcause some of the solution to run and drip off the cat. If a second spot is needed to avoid run off, then apply thesecond spot slightly behind the first spot.
Treatment with Bravecto may begin at any time of the year and can continue year round without interruption.

The potential risks ticks pose to pets can be stressful to pet owners wanting to ensure their pet receives effective coverage. Bravecto kills 4 different species of ticks commonly found on dogs and 2 species found on cats to protect your pet for 12 weeks.*5-7
PROTECT YOUR PET AGAINST THESE TICK SPECIES5-7

Contraindications:
There are no known contraindications for the use of the product.
Warnings:
Human Warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not contact or allow children to contact the application site until dry.
Keep the product in the original packaging until use in order to prevent children from getting direct access to the product. Do not eat, drink or smoke while handling the product. Avoid contact with skin and eyes. If contact with eyes occurs, then flush eyes slowly and gently with water. Wash hands and contacted skin thoroughly with soap and water immediately after use of the product.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
Precautions:
For topical use only. Avoid oral ingestion. (see Animal Safety).
Use with caution in cats with a history of neurologic abnormalities. Neurologic abnormalities have been reported in cats receiving Bravecto, even in cats without a history of neurologic abnormalities (see Adverse Reactions).
Bravecto has not been shown to be effective for 12-weeks duration in kittens less than 6 months of age. Bravecto is not effective against Dermacentor variabilis ticks beyond 8 weeks after dosing (see Effectiveness).
The safety of Bravecto has not been established in breeding, pregnant and lactating cats.
Adverse Reactions:
In a well-controlled U.S. field study, which included a total of 161 households and 311 treated cats (224 with fluralaner and 87 with a topical active control), there were no serious adverse reactions.
Percentage of Cats with Adverse Reactions (AR) in the Field Study
| Adverse Reaction (AR) | Bravecto Group: Percent of Cats with the AR During the 105-Day Study (n=224 cats) | Control Group: Percent of Cats with the AR During the 84-Day Study (n=87 cats) |
|---|---|---|
| Vomiting | 7.6% | 6.9% |
| Pruritus | 5.4% | 11.5% |
| Diarhea | 4.9% | 1.1% |
| Alopecia | 4.9% | 4.6% |
| Decreased Appetite | 3.6% | 0.0% |
| Lethargy | 3.1% | 2.3% |
| Scabs/Ulcerated Lesions | 2.2% | 3.4% |
In the field study, two cats treated with fluralaner topical solution experienced ataxia. One cat became ataxic with a right head tilt 34 days after the first dose. The cat improved within one week of starting antibiotics. The ataxia and right head tilt, along with lateral recumbency, reoccurred 82 days after administration of the first dose. The cat recovered with antibiotics and was redosed with fluralaner topical solution 92 days after administration of the first dose, with no further abnormalities during the study. A second cat became ataxic 15 days after receiving its first dose and recovered the next day. The cat was redosed with fluralaner topical solution 82 days after administration of the first dose, with no further abnormalities during the study.
In a European field study, two cats from the same household experienced tremors, lethargy, and anorexia within one day of administration. The signs resolved in both cats within 48-72 hours.
In a European field study, there were three reports of facial dermatitis in humans after close contact with the application site which occurred within 4 days of application.
For technical assistance or to report a suspected adverse drug reaction, or to obtain a copy of the Safety Data Sheet (SDS), contact Merck Animal Health at 1-800-224-5318. Additional information can be found at www.bravecto.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Clinical Pharmacology:
Peak fluralaner concentrations are achieved between 7 and 21 days following topical administration and the elimination half-life ranges between 11 and 13 days.
Mode of Action:
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
Effectiveness:
In a well-controlled European laboratory study, Bravecto killed 100% of fleas 8 hours after treatment and reduced the number of live fleas on cats by > 98% within 12 hours after treatment or post-infestation for 12 weeks. In well-controlled laboratory studies, Bravecto demonstrated > 94% effectiveness against Ixodes scapularis 48 hours post- infestation for 12 weeks. Bravecto demonstrated > 98% effectiveness against Dermacentor variabilis 48 hours post-infestation for 8 weeks, but failed to demonstrate ≥ 90% effectiveness beyond 8 weeks.
In a well-controlled U.S. field study, a single dose of Bravecto reduced fleas by ≥99% for 12 weeks. Cats with signs of flea allergy dermatitis showed improvement in erythema, alopecia, papules, scales, crusts, and excoriation as a direct result of eliminating flea infestations.
Animal Safety:
Margin of Safety Study: In a margin of safety study, Bravecto was administered topically to 11- to 13-week (mean age 12 weeks)-old-kittens at 1, 3, and 5X the maximum labeled dose of 93 mg/kg at three, 8-week intervals (8 cats per group). The cats in the control group (0X) were treated with mineral oil.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Cosmetic changes at the application site included matting/clumping/spiking of hair, wetness, or a greasy appearance.
Oral Safety Study: In a safety study, one dose of Bravecto topical solution was administered orally to 6 - to 7-month-old- kittens at 1X the maximum labeled dose of 93 mg/kg. The kittens in the control group (0X) were administered saline orally. There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. All treated kittens experienced salivation and four of six experienced coughing immediately after administration. One treated kitten experienced vomiting 2 hours after administration.
In a well-controlled field study Bravecto was used concurrently with other medications, such as vaccines, anthelmintics, antibiotics, steroids and sedatives. No adverse reactions were observed from the concurrent use of Bravecto with other medications.
Storage
Do not store above 77°F (25°C). Store in the original package in order to protect from moisture. The pouch should only be opened immediately prior to use.
FAQ
Bravecto has a wide margin of safety in dogs who weigh at least 4.4 lb. and cats who weigh at least 2.6 lb. It is also approved for puppies and kittens aged 6 months or older.1-3
Bravecto Chew is approved for use in breeding, pregnant, and lactating dogs.1,4
Bravecto is the only flea and tick treatment that lasts 12 weeks* with 1 convenient chew or topical dose.1-3 Compared to any monthly treatment, your pet is protected nearly 3 times longer with Bravecto.
The long-lasting protection of Bravecto effectively eliminates flea infestations on pets with just 1 dose.7,8 Plus, Bravecto kills ticks faster-killing 100% of attached ticks in 12 hours. Other treatments can take over 48 hours.9
Bravecto Chew starts killing fleas (Ctenocephalides felis) within 2 hours, and kills ticks (Ixodes ricinus) within 12 hours.*1 Bravecto Chew kills fleas, prevents flea infestations, and kills ticks (black-legged tick, American dog tick, and brown dog tick) for 12 weeks. Bravecto Chew also kills lone star ticks for 8 weeks.1
Bravecto Topical Solution for Dogs reduces fleas by ≥99% and 100% within 24 and 48 hours, respectively, post-infestation for 12 weeks.* It kills ≥93.3% of black-legged ticks, American dog ticks and brown dog ticks after 48 hours for 12 weeks* and kills ≥90% lone star ticks 72 hours post-infestation for 8 weeks.2
Bravecto Topical Solution for Cats kills 100% of fleas within 8 hours. Applied to the base of the skull, your cat will have full-body protection for 12 weeks*-killing fleas, preventing flea infestations, and killing black-legged ticks. Bravecto Topical Solution for Cats also kills American dog ticks for 8 weeks.3
Your cat doesn't need to go outside to be at risk for fleas and ticks. Cats can be exposed to fleas and ticks from dogs in your house. However, even cats without dog siblings can be at risk. You can carry fleas inside on your clothes and shoes - and the larvae and pupae can hide within your home for weeks before hopping on your cat.
If you live in an apartment complex, fleas can move from 1 apartment to the next. And, certain ticks can survive indoors throughout their lifecycle, so they can come in with you from outside and eventually attach to your pet.10
Reviews
- Sean
- Cost of product bravecto
- My product arrrived when Entirely Pets stated it would be shipped.
- Very pleased with my order
- Works great!!!
- Everything I already wrote!
- I haven
- The price on EntirelyPets Pharmacy is the best I could find anywhere, especially with 20% off.
- It works.
- It's expensive!
- Once every 3 months!
- Great for flees
- Effective!
- Very tiny tube of a concentrated product. Don't spill a drop!
- Lasts 12 weeks
- Easy to administer
- Lasts for 3 months
- Keeps the fleas away better than any other flea
- control I have used in the last 14 years
- Easy to use






















![Bravecto Topical Solution for Cats 6.3-13.8 lbs (4 tubes)|12 Months Protection - [Flea & Tick] Video](https://img.youtube.com/vi/sZN_XgdZqBc/0.jpg)
![Bravecto Topical Solution for Cats 6.3-13.8 lbs (4 tubes)|12 Months Protection - [Flea & Tick] Video](https://img.youtube.com/vi/vU_aii5AM6E/0.jpg)







