SAVE 17% OFF 17% OFF Use Code LUCKY17 *
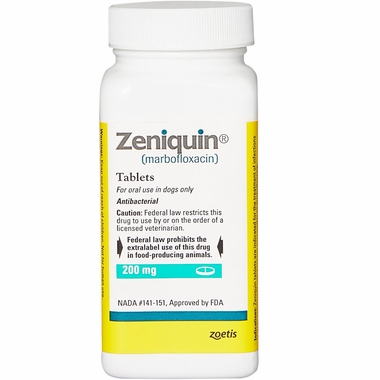
Zeniquin for Dogs & Cats - 200 mg (Per Tablet) - [Bacterial Infections Treatment]
- Description
- Directions
- FAQ
- Reviews
Description
Zeniquin is a broad spectrum antibiotic used for the treatment of bacterial infections in dogs and cats, such as skin and soft-tissue infections and urinary tract infections due to organisms susceptible to marbofloxacin. The tablets are film-coated and easy for your pet to swallow.
Key Benefits
- Zeniquin has the highest area under the curve (AUC) compared with all other veterinary-approved fluoroquinolones.
- Zeniquin has a longer half-life than any other veterinary-approved fluoroquinolone.
- Target animal safety studies in cats did not demonstrate inducible dose-related ocular toxicity.
- Convenient once-a-day dosing.
- Scored and film-coated tablets are available in 4 sizes, providing flexible dosing for small to giant-sized breeds.
How It Works
Zeniquin is a fluoroquinolone antibiotic that works by inhibiting bacterial DNA replication.
Indications
Zeniquin (marbofloxacin) tablets are indicated for the treatment of infections in dogs and cats associated with bacteria susceptible to marbofloxacin.
Directions
View Vetoryl Drug Facts Sheet.
- Zeniquin is a prescription broad-spectrum oral antibiotic used in dogs and cats for the treatment of bacterial infections such as skin and soft-tissue infections and urinary tract infections due to susceptible organisms.
- Give this medication exactly as directed by your veterinarian.
Tip: Zeniquin should not be used in cats younger than 12 months of age or dogs during their rapid growth phase, which can vary from 8-18 months based on the breed (small and medium breeds up to 8 months of age, large breeds up to 12 months of age, and giant breeds up to 18 months of age).
| Zeniquin Dosage for Cats | |
|---|---|
| Weight | Dosage |
| All weights | The usual dose 1.25 mg/lb of pet's body weight given once a day, but the dosage may be increased to 2.5 mg/lb of pet's body weight. For treatment of skin and soft tissue infections, give for 2-3 days after symptoms are gone, for a maximum of 30 days. For treatment of urinary tract infections, give for at least 10 days. However, if there is no improvement after 5 days your pet should be reevaluated. |
| Zeniquin Dosage for Dogs | |
| Weight | Dosage |
| All weights | The usual dose 1.25 mg/lb of pet's body weight given once a day, but the dosage may be increased to 2.5 mg/lb of pet's body weight. For treatment of skin and soft tissue infections, give for 2-3 days after symptoms are gone, for a maximum of 30 days. For treatment of urinary tract infections, give for at least 10 days. However, if there is no improvement after 5 days your pet should be reevaluated. |
Cautions:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Contraindications:
Marbofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested, the dog being par-ticularly sensitive to this side effect. Marbofloxacin is contraindicated in immature dogs during the rapid growth phase (small and medium breeds up to 8 months of age, large breeds up to 12 months of age and giant breeds up to 18 months of age). Marbofloxacin is contraindicated in cats under 12 months of age. Marbofloxacin is contraindicated in dogs and cats known to be hypersensitive to quinolones.
Warning:
For use in animals only. Keep out of reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposure. Individuals with a history of hypersensitivity to fluoroquinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
Precautions:
Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. In such animals, quino-lones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolones have been shown to produce erosionsof cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species. The use of fluoroquinolones in cats has been reported to adversely affect the retina. Such products should be used with caution in cats. The safety of marbofloxacin in animals used for breeding purposes, pregnant, or lactating has not been demonstrated.
Adverse Reactions:
The following clinical signs were reported during the course of clinical field studies in dogs receiving marbofloxacin at dosages up to 2.5 mg/lb daily: decreased or loss of appetite (5.4%), decreased activity (4.4%), and vomiting (2.9%). The following signs were reported in less than 1% of cases in dogs: increased thirst, soft stool/diarrhea, behavioral changes, shivering/shaking/tremors, and ataxia. One dog which had a seizure the day before study enrollment experienced a seizure while on marbofloxacin therapy.
The following clinical signs were reported during clinical field studies in cats receiving 1.25 mg/lb/day: diarrhea (2.1%) and soft stool (1.4%). Vomiting was reported in less than 1% of cases in cats.
Storage:
Store below 30°C (8°F).
FAQ
Reviews
- Many
- Few to none