SAVE 20% OFF 20% OFF Use Code PREZ20 *
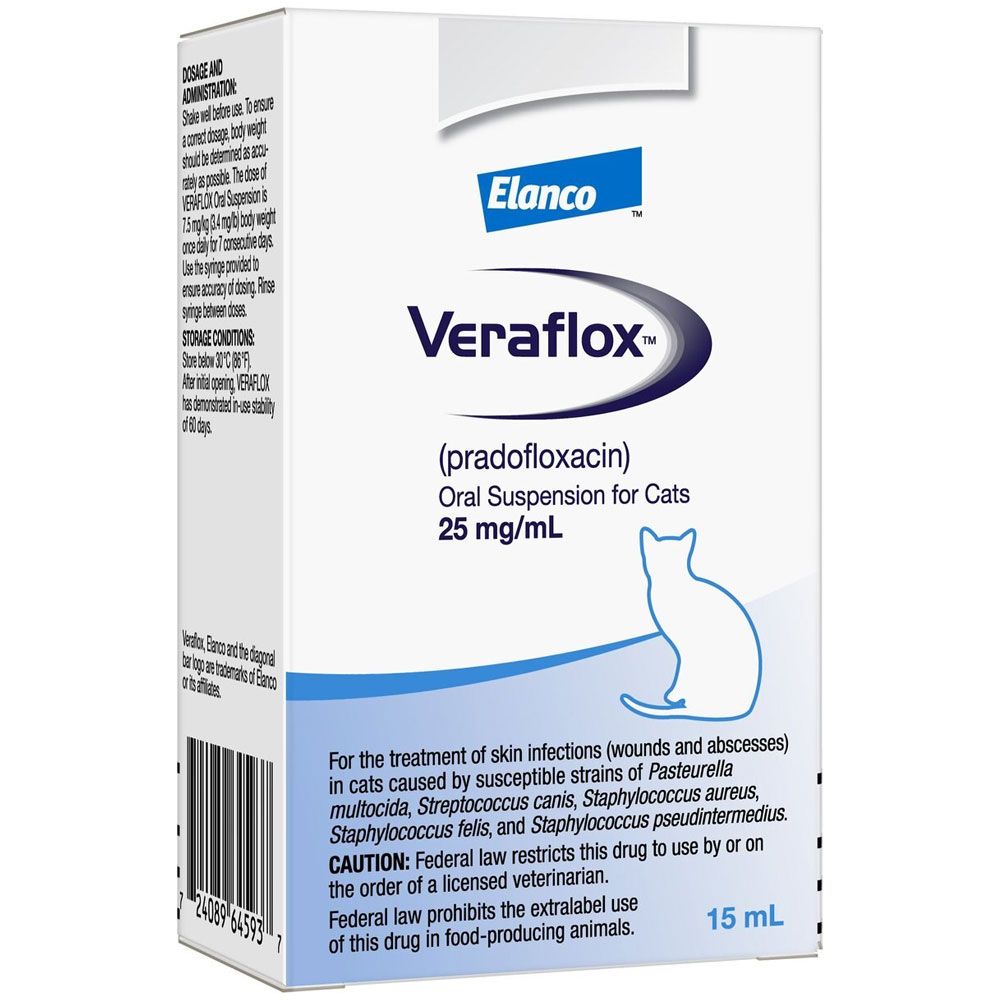
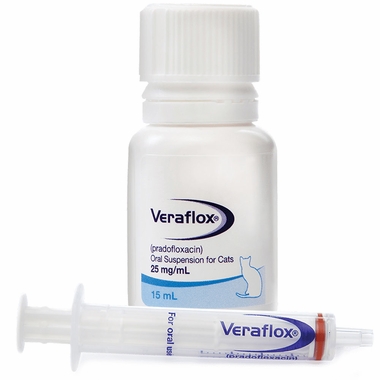
VerafloxCats Oral Suspension for Cats 2.5% 15ml - [Skin Infection Treatment]
- Notice
- Description
- Directions
- FAQ
- Reviews
Notices
ON SALE! For a limited time. Add to cart to see automatic coupon. We have the best price and service on the internet.
Description
Veraflox is an oral solution for cats that treats skin infections. This antibiotic helps your cat heal from infections from wounds and abscesses caused by susceptible strains of Gram-negative, Gram-positive, and anaerobic bacteria (Pasteurella multocida, Streptococcus canis, Staphylococcus aureus, Staphlyococcus felis and Staphylococcus pseudintermedius).
Key Benefits
- Treats skin infections from wounds and abscesses
- Effective against Gram-negative, Gram-positive, and anaerobic bacteria
- Easy to administer with included dosing syringe
How It Works
Veraflox works by attacking the essential target enzymes needed for the reproduction of Gram-positive, Gram-negative, and anaerobic bacteria.
Indications
Veraflox is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida, Streptococcus canis, Staphylococcus aureus, Staphylococcus felis, and Staphylococcus pseudintermedius.
Directions
View Veraflox Drug Facts Sheet.
Shake well before use. To ensure a correct dosage, body weight should be determined as accurately as possible. The dose of VERAFLOX is 7.5 mg/kg (3.4 mg/lb) body weight once daily for 7 consecutive days. Use the syringe provided to ensure accuracy of dosing to the nearest 0.1 mL. Rinse syringe between doses.
A sample of the lesion should be obtained for culture and susceptibility testing prior to beginning antibacterial therapy. Once results become available, continue with appropriate therapy. If acceptable response to treatment is not observed, or if no improvement is seen within 3 to 4 days, then the diagnosis should be re-evaluated and appropriate alternative therapy considered.
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Contraindications:
DO NOT USE IN DOGS. Pradofloxacin has been shown to cause bone marrow suppression in dogs. Dogs may be particularly sensitive to this effect, potentially resulting in severe thrombocytopenia and neutropenia.
Quinolone-class drugs have been shown to cause arthropathy in immature animals of most species tested, the dog being particularly sensitive to this side effect.
Pradofloxacin is contraindicated in cats with a known hypersensitivity to quinolones.
Warnings:
Human Warnings:
Not for human use. Keep out of reach of children.
Individuals with a history of quinolone hypersensitivity should avoid this product. Avoid contact with eyes and skin. In case of ocular contact, immediately flush eyes with copious amounts of water. In case of dermal contact, wash skin with soap and water immediately for at least 20 seconds. Consult a physician if irritation persists following ocular or dermal exposure, or in case of accidental ingestion. In humans, there is a risk of photosensitization within a few hours after exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight. Do not eat, drink or smoke while handling this product.
It is recommended that used syringes be kept out of reach of children and disposed of properly.
Animal Warnings:
For use in cats only.
The administration of pradofloxacin for longer than 7 days induced reversible leukocyte, neutrophil, and lymphocyte decreases in healthy, 12-week-old kittens (see Animal Safety section). If an unexplained drop in leukocyte, neutrophil, and/or lymphocyte counts is noted during pradofloxacin therapy, discontinuation of treatment is recommended.
Precautions:
Prescribing antibacterial drugs in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant animal pathogens.
The use of fluoroquinolones in cats has been associated with the development of retinopathy and/or blindness. Such products should be used with caution in cats.
Quinolones have been shown to produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species.
The safety of pradofloxacin in immune-compromised cats (i.e., cats infected with feline leukemia virus and/or feline immunodeficiency virus) has not been evaluated.
Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation that may lead to convulsive seizures.
The safety of pradofloxacin in cats younger than 12 weeks of age has not been evaluated.
The safety of pradofloxacin in cats that are used for breeding or that are pregnant and/or lactating has not been evaluated.
DRUG INTERACTIONS: Compounds (e.g., sucralfate, antacids and multivitamins) containing divalent and trivalent cations (e.g., iron, aluminum, calcium, magnesium, and zinc) may substantially interfere with the absorption of quinolones resulting in a decrease in product bioavailability. Therefore, the concomitant oral administration of quinolones with foods, supplements, or other preparations containing these compounds should be avoided.
The dosage of theophylline should be reduced when used concurrently with quinolones. Cimetidine has been shown to interfere with the metabolism of quinolones and should be used with care when used concurrently. Concurrent use of quinolones with oral cyclosporine should be avoided. Concurrent administration of quinolones may increase the action of oral anticoagulants.
Storage:
Store below 30°C (86°F).
After initial opening, VERAFLOX has demonstrated in-use stability of 60 days.