SAVE 15% OFF 15% OFF Use Code EPX15 *

Meloxidyl (meloxicam) for Dogs - Oral Suspension 1.5 mg/mL, 10-mL - [Pain & Inflammation Relief]
- Notice
- Description
- Directions
- Reviews
Notices
This is our Rock-Bottom-Price! Sorry, Promotional offers do not apply (See Exclusions.)
Description
Meloxidyl (meloxicam) is a nonsteroidal anti-inflammatory medication prescribed by your vet to help reduce pain, inflammation, and stiffness and increase mobility in your dog. It helps treat inflammation and may also help control pain associated with severe and chronic diseases, such as arthritis. Because it comes in liquid form, you can give to your dog directly by mouth or simply disguise it in his favorite wet food. Customers may receive this drug under the name Metacam, Meloxidyl, Loxicom.
Uses
For the control of pain and inflammation associated with arthritis or other conditions causing muscle soreness or stiffness in dogs.
Possible Side Effects
Typical adverse reactions to non-steroidal anti-inflammatories may include vomiting, diarrhea, lethargy, decreased appetite and behavioral changes. In cases of anaphylaxis, you should consult your veterinarian immediately.
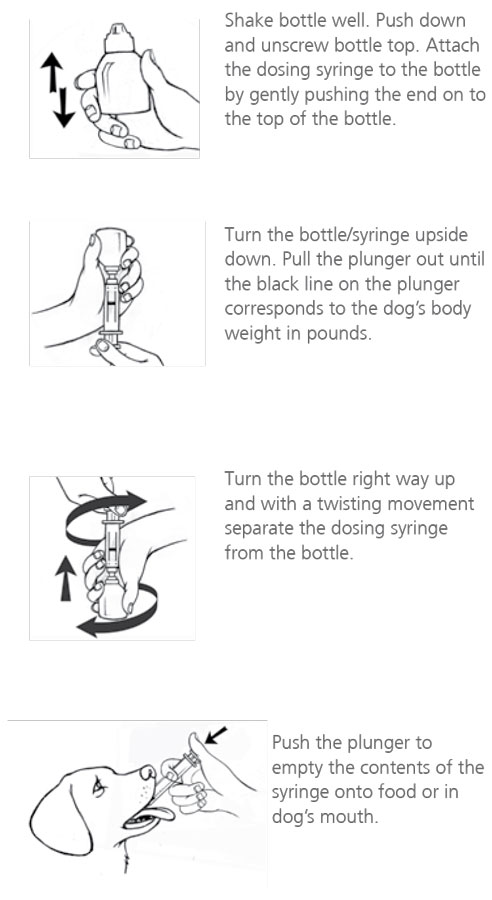
Directions
View Meloxidyl Drug Facts Sheet.
Meloxidyl Oral Suspension is packaged with 2 sizes of dosing syringes. The small syringe (blue print) is calibrated for use in dogs under 15 lbs. The large syringe (green print) is calibrated for use in dogs 15 lbs or greater. Only administer Meloxidyl with the provided syringes. The container should never be used as a dropper bottle for administration of Meloxidyl.
Dogs under 15 lbs (6.8 kg)
Shake well before use, then remove cap. Meloxidyl Oral Suspension can be given either mixed with food or placed directly into the mouth. Particular care should be given with regard to the accuracy of dosing.
To prevent accidental overdosing of small dogs, only use the small dosing syringe. The large syringe provided cannot be used to measure doses for dogs weighing less than 15 lbs (6.8 kg). For dogs less than 15 lbs, use the small dosing syringe (blue print) provided in the package (see dosing procedure below).
The small dosing syringe fits onto the bottle and has dosing marks at 0.5 lb, then in 1 lb increments (ranging from 1 to 14 lbs), designed to deliver the daily maintenance dose of 0.05 mg/lb (0.1 mg/kg).
For dogs less than 1 lb (0.45 kg), Meloxidyl can be given using the 0.5 mark on the small dosing syringe.
For dogs between 1 - 14 lbs, Meloxidyl can be given using the marks on the small dosing syringe, beginning at 1 lb and ending at 14 lbs. When using the small dosing syringe, the dog’s weight should be rounded down to the nearest 1 lb increment. Replace and tighten cap after use.
Dogs 15 lbs (6.8 kg) and over
Shake well before use, then remove cap. Meloxidyl may be either mixed with food or placed directly into the mouth. Particular care should be given with regard to the accuracy of dosing.
For dogs 15 lbs or greater, use the large dosing syringe (green print) provided in the package (see dosing procedure below). The large dosing syringe fits on to the bottle and has dosing marks in 5 lb increments (ranging from 5 to 140 lbs), designed to deliver the daily maintenance dose of 0.05 mg/lb (0.1 mg/kg). When using the large syringe, the dog’s weight should be rounded down to the nearest 5 lb increment. Replace and tighten cap after use.
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Contraindications:
Dogs with known hypersensitivity to meloxicam should not receive Meloxidyl Oral Suspension. Do not use Meloxidyl Oral Suspension in cats. Acute renal failure and death have been associated with the use of meloxicam in cats.
Warning:
Repeated use of meloxicam in cats has been associated with acute renal failure and death. Do not administer additional injectable or oral meloxicam to cats. See Contraindications, Warnings, and Precautions for detailed information.
Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental ingestion by humans. For oral use in dogs only.
As with any NSAID, all dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory testing to establish hematological and serum biochemical baseline data is recommended prior to and periodically during administration. Owner should be advised to observe their dog for signs of potential drug toxicity and be given a client information sheet about Meloxidyl Oral Suspension.
Precautions:
The safe use of Meloxidyl Oral Suspension in dogs younger than 6 months of age, dogs used for breeding, or in pregnant or lactating dogs has not been evaluated. Meloxicam Oral Suspension is not recommended for use in dogs with bleeding disorders, as safety has not been established in dogs with these disorders. As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such antiprostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since NSAIDs possess the potential to induce gastrointestinal ulcerations and/or perforations, concomitant use with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. If additional pain medication is needed after administration of the total daily dose of Meloxidyl Oral Suspension, a non-NSAID or non-corticosteroid class of analgesia should be considered. The use of another NSAID is not recommended. Consider appropriate washout times when switching from corticosteroid use or from one NSAID to another in dogs. The use of concomitantly protein-bound drugs with Meloxidyl Oral Suspension has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of Meloxidyl Oral Suspension has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
Adverse Reactions:
Field safety was evaluated in 306 dogs. Based on the results of two studies, GI abnormalities (vomiting, soft stools, diarrhea, and inappetance) were the most common adverse reactions associated with the administration of meloxicam. The following table lists adverse reactions and the numbers of dogs that experienced them during the studies. Dogs may have experienced more than one episode of the adverse reaction during the study.
In foreign suspected adverse drug reaction (SADR) reporting over a 9 year period, incidences of adverse reactions related to meloxicam administration included: auto-immune hemolytic anemia (1 dog), thrombocytopenia (1 dog), polyarthritis (1 dog), nursing puppy lethargy (1 dog), and pyoderma (1 dog).
Effectiveness:
The effectiveness of meloxicam was demonstrated in two field studies involving a total of 277 dogs representing various breeds, between six months and sixteen years of age, all diagnosed with osteoarthritis. Both of the placebo-controlled, masked studies were conducted for 14 days. All dogs received 0.2 mg/kg on day 1. All dogs were maintained on 0.1 mg/kg oral meloxicam from days 2 through 14 of both studies. Parameters evaluated by veterinarians included lameness, weight-bearing, pain on palpation, and overall improvement. Parameters assessed by owners included mobility, ability to rise, limping, and overall improvement. In the first field study (n= 109), dogs showed clinical improvement with statistical significance after 14 days of meloxicam treatment for all parameters. In the second field study (n = 48), dogs receiving meloxicam showed a clinical improvement after 14 days of therapy for all parameters; however, statistical significance was demonstrated only for the overall investigator evaluation on day 7, and for the owner evaluation on day 14.
Storage:
Store at controlled room temperature 68-77° F (20-25° C).


