SAVE 20% OFF 20% OFF Use Code PREZ20 *

Intralipid 20% IV Fat Emulsion - Sterile BioFine Container ( 500 ml)
- Description
- Directions
- Reviews
Description
Intralipid 20% ( A 20% Intravenous Fat Emulsion) is indicated as a source of calories and essential fatty acids for patients requiring parenteral nutrition for extended periods of time (usually for more than 5 days) and as a source of essential fatty acids for prevention of Essential Fatty Acid Deficiency (EFAD).
Intralipid 20% is not intended for direct infusion. It is a sterile dosage form which contains several single doses for use in the preparation of three-in-one or total nutrient admixtures (TNAs) in a pharmacy admixture program.
Intralipid is metabolized and utilized as a source of energy causing an increase in heat production, decrease in respiratory quotient and increase in oxygen consumption. The infused fat particles are cleared from the blood stream in a manner thought to be comparable to the clearing of chylomicrons.
Intralipid will prevent the biochemical lesions of essential fatty acid deficiency (EFAD), and correct the clinical manifestations of the EFAD syndrome.
Key Benefits
- The container system is composed of a clear plastic oxygen-barrier overwrap.
- Between the overwrap and the inner Intralipid I.V. Fat Emulsion container are an oxygen absorber and the OXALERT Integrity Indicator.
- Non-DEHP lipid-compatible polypropylene container
- Does not contain latexnClear plastic overwrap protects the product from the environment
- Bar-coded labeling
- Easy to handle and lighter than glass bottles
- Helps to eliminate waste from glass breakage
- Reduces the need for vented administration sets
Indications
Intralipid 20% (A 20% Intravenous Fat Emulsion) is indicated as a source of calories and essential fatty acids for patients requiring parenteral nutrition for extended periods of time (usually for more than 5 days) and as a source of essential fatty acids for prevention of Essential Fatty Acid Deficiency (EFAD).
Intralipid 20% is indicated for use in a pharmacy admixture program for the preparation of three-in-one or total nutrition admixtures (TNAs) to provide a source of calories and essential fatty acids for patients requiring parenteral nutrition for extended periods of time (usually for more than 5 days) and a source of essential fatty acids for prevention of EFAD.
Directions
Intralipid 20% (A 20% I.V. Fat Emulsion) should be administered only as a part of a three-in-one or total nutrient admixture via peripheral vein or by central venous infusion.
Intralipid 20% IS NOT INTENDED FOR DIRECT INFUSION. The container closure may be penetrated only once using a suitable sterile transfer device or dispensing set which allows measured dispensing of the contents. The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area). Once the closure is penetrated, the contents should be dispensed as soon as possible; the transfer of contents to suitable TPN admixture containers must be completed within 4 hours of closure penetration. The bag should be stored below 25°C (77°F) after the closure has been entered. Admixtures made using Intralipid 20% should be used promptly.
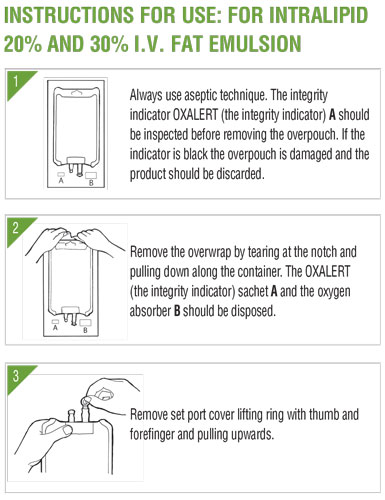
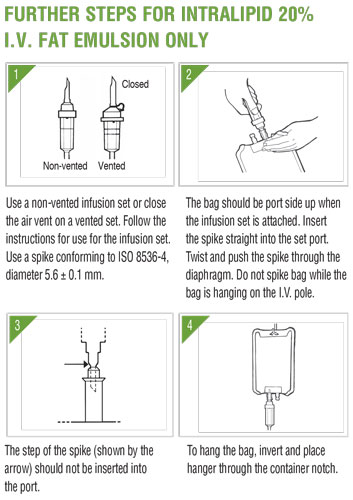
Important Risk Information
Warnings:
Deaths in preterm infants after infusion of intravenous fat emulsion have been reported in the medical literature. Autopsy findings included intravascular fat accumulation in the lungs. Treatment of premature and low birth weight infants with intravenous fat emulsion must be based upon careful benefit-risk assessment. Strict adherence to the recommended total daily dose is mandatory; hourly infusion rate should be as slow as possible in each case and should not in any case exceed 1 g fat/kg in 4 hours. Premature and small-for-gestational age infants have poor clearance of intravenous fat emulsion and increased free fatty acid plasma levels following fat emulsion infusion; therefore, serious consideration must be given to administration of less than the maximum recommended doses in these patients in order to decrease the likelihood of intravenous fat overload. The infant's ability to eliminate the infused fat from the circulation must be carefully monitored (such as serum triglycerides and/or plasma free fatty acid levels). The lipemia must clear between daily infusions.
- Intralipid 30% Pharmacy Bulk Package is not intended for direct intravenous administration. Diluting Intralipid 30% to a 10% or 20% concentration with an IV fluid diluent does not produce a dilution that is equivalent in composition to Intralipid 10% or 20% IV fat emulsions and should not be given by direct IV administration. The administration of Intralipid 20% and Intralipid 30% is contraindicated in patients with disturbances of normal fat metabolism such as pathologic hyperlipemia, lipoid nephrosis or acute pancreatitis if accompanied by hyperlipidemia.
- Exercise caution when administering Intralipid 20% and Intralipid 30% to patients with severe liver damage, pulmonary disease, anemia or blood coagulation disorders, or when there is danger of fat embolism.
- Intralipid 20% and Intralipid 30% containsaluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is mpaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions which contain aluminum.
- Monitor serum triglycerides to determine the patient's capacity to eliminate the infused fat from the circulation. Overdosage must be avoided. During long-term intravenous nutrition with Intralipid 20% and Intralipid 30%, liver function tests should be performed. If these tests indicate that liver function is impaired, the therapy should be withdrawn.
- Those more frequently encountered adverse events are due: either to contamination of the IV catheter and result in sepsis, or to vein irritation by concurrently infused hypertonic solutions and may result in thrombophlebitis. These adverse reactions are inseparable from the hyperalimentation procedure with or without Intralipid 20% and Intralipid 30%.
- Frequent adverse events include sepsis due to contamination of the IV catheter and vein irritation may result in thrombophlebitis by concurrently infused hypertonic solutions. Less frequent adverse reactions more directly related to Intralipid 20% and Intralipid 30% reported <1% in clinical trials are:
- Immediate or early: dyspnea, cyanosis, allergic reactions, hyperlipemia, hypercoagulability, nausea, vomiting, headache, flushing, increase in temperature, sweating, sleepiness, pain in the chest and back, slight pressure over the eyes, dizziness, and irritation at the site of infusion and rarely thrombocytopenia in neonates.
- Delayed: hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, transient increases in liver function tests, and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
- The deposition of a brown pigmentation in the reticuloendothelial system called “intravenous fat pigment” has been reported. The causes and significance are unknown.


