SAVE 20% OFF 20% OFF Use Code PREZ20 *

Centragard for Small Cats - for Small Cats (1 Month) - [Heartworm Prevention]
- Description
- Ingredients
- Directions
- Reviews
Description
Centragard is a topical solution for cats that provides protection against heartworm disease caused by Dirofilaria immitis and treats and controls roundworms, tapeworms, and hookworms. It is a safe, monthly topical medication for cats and kittens 7 weeks of age or older and 5.6 lbs or more. Centragard comes in single-use applicators for simple administration. Centragard requires a prescription from your veterinarian.
Key Benefits
- Protects your cat from heartworm disease
- Treats and controls roundworms, tapeworms, and hookworms
- Only one dose required per month
- Easy to administer.
How It Works
When applied, Centragard absorbs through the skin and circulates into the bloodstream. Concentrations of eprinomectin and praziquantel, the active ingredients, in the tissue and bloodstream help prevent heartworm disease and treat and control other worms.
Indications
Centragard is indicated for the prevention of heartworm disease caused by Dirofilaria immitis, and for the treatment and control of roundworms (adult and fourth stage larval Toxocara cati), hookworms (adult and fourth stage larval Ancylostoma tubaeforme; adult Ancylostoma braziliense), and tapeworms (adult Dipylidium caninum and Echinococcus multilocularis) in cats and kittens 7 weeks of age and older and 1.8 lbs or greater.
Directions
View Centragard Drug Facts Sheet.
Centragard is dosed at a minimum of 0.055 mL/lb (0.12 mL/kg), which delivers a minimum dose of 0.23 mg/lb eprinomectin and 4.55 mg/lb praziquantel. Administer the entire contents of a Centragard unit applicator topically once a month as specfied in the following table:
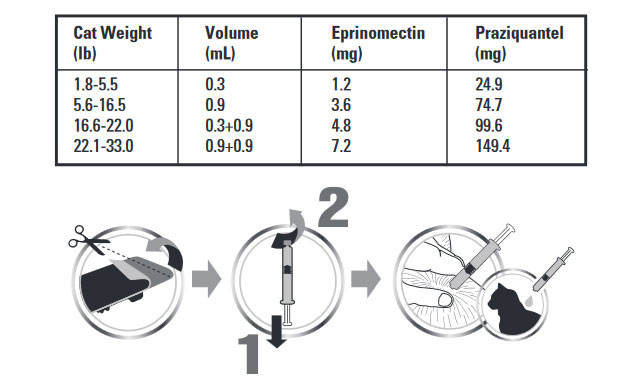
To apply Centragard pull back the plunger of the unit applicator slightly and remove the cap. Part the hair in one spot on the midline of the neck between the base of the skull and the shoulder blades, place the tip of the unit applicator on the skin and apply the contents directly on the skin. If the weight of the cat requires a second application, apply the contents in the same manner as described above in the same location.Discard applicator after use.
Heartworm Prevention:
For prevention of heartworm disease, Centragard should be administered once a month. Centragard may be administered year round or at a minimum, should start 1 month before the cat's first expected exposure to mosquitoes and continuing at monthly intervals until at least one month after the cat's last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Centragard immediately and resume the monthly dosing schedule.
When replacing another monthly heartworm preventive product in a heartworm prevention program, the first treatment with Centragard should be given within one month of the last dose of the former medication. At the discretion of the veterinarian, cats older than 6 months of age may be tested to determine the presence of existing heartworm infection before treatment with Centragard.
Treatment and Control of Roundworms, Hookworms and Tapeworms:
Centragard treats and controls roundworms (adult and fourth stage larval Toxocara cati), hookworms (adult and fourth stage larval Ancylostoma tubaeformae, adult Ancylostoma braziliense), and tapeworms (adult Dipylidium caninum and Echinococcus multilocularis) after a single administration or when given monthly as part of a heartworm prevention program. Cats may be exposed to and can become infected with roundworms, hookworms, and tapeworms throughout the year, regardless of season or climate. Clients should be advised of appropriate measures to prevent reinfection of their cat with intestinal parasites. Because the prepatent period for E. multilocularis may be as short as 26 days, cats treated at the labeled monthly intervals may become reinfected and shed eggs between treatments.
Human Warning:
Not for human use. Keep out of reach of children. Avoid contact with the application site for 5 hours following treatment. Wash hands after administering the product. If the product accidentally gets into the eyes, flush thoroughly with water. In case of accidental ingestion, or if skin or eye irritation occurs, contact a poison control center or physician for treatment advice.
Precautions:
Do not administer orally. Cats may salivate excessively and vomit if Centragard is accidentally administered orally or is ingested through licking/grooming the application site (see Animal Safety).The safety of Centragard has not been tested in breeding, pregnant or lactating cats.The safety of Centragard has not been tested in kittens less than 7-9 weeks of age or weighing less than 1.8 lbs (0.8 kg).
Adverse Reactions:
In a well-controlled field study emesis, anorexia, lethargy, temporary clumping or spiking of the hair, or mild, transient skin reactions (itching, hair loss) were reported. When cats licked the application site after treatment, temporary excessive salivation was observed. Oral ingestion of Centragard may also result in hypersalivation, vomiting and/or lethargy. In margin of safety studies, transient neurological signs such as ataxia, disorientation, lethargy, and pupil dilation were observed in some cats. Correct application will minimize the occurrence of such events.
To report suspected adverse events, for technical assistance or to obtain a copy of the SDS, contact Merial at 1-888-637-4251.
For additional information about adverse drug experience reporting for animal drugs,contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/-SafetyHealth.
The Safety Data Sheet (SDS) provides additional occupational safety information. For customer service or to obtain product information, including the SDS, call 1-888-637-4251.
Information for Owner or Person Treating Animal:
Echinococcus multilocularis is a tapeworm found in wild canids and domestic cats. E. multilocularis can infect humans and cause serious disease (alveolar hydatid disease). Owners of cats living in areas where E. multilocularis are endemic should be instructed on how to minimize their risk of exposure to this parasite, as well as their cat's risk of exposure. Although ML-635 was 100% effective in laboratory studies in cats against E. multilocularis, no studies have been conducted to show that the use of this product will decrease the incidence of alveolar hydatid disease in humans. Because the prepatent period for E. multilocularis may be as short as 26 days, cats treated at the labeled monthly intervals may become reinfected and shed eggs between treatments.
Mode of Action:
Eprinomectin binds to glutamate gated chloride channels that are present in invertebrate nerve and muscle cells and increases the permeability of the cell membrane to chloride ions that triggers hyperpolarization of the nerve or muscle cell resulting in paralysis and death of the parasite.
Praziquantel's mode of action is not precisely known but treated tapeworms undergo muscular paralysis accompanied by a rapid influx of calcium ions and the disruption of the tegument.
Effectiveness:
Effectiveness studies were conducted with an early formulation (ML-635), containing 8.3% fipronil, 0.4% eprinomectin, 8.3% praziquantel, and 10% (S)-methoprene. The doses of eprinomectin and praziquantel in ML-635 are equivalent to the final formulation of Centragard (eprinomectin and praziquantel transdermal solution).
Heartworm Disease Prevention:
In well-controlled laboratory studies, ML-635 provided 100% effectiveness against induced heartworm infections after a single application.
Treatment and Control of Roundworms, Hookworms, and Tapeworms:
In well-controlled laboratory studies, ML-635 provided >90% effectiveness against natural and/or induced roundworm (adult and fourth stage larval Toxocara cati); hookworm (adult and fourth stage larval Ancylostoma tubaeforme; adult Ancylostoma braziliense), and adult tapeworm (Dipylidium caninum; Echinococcus multilocularis) infections.
Animal Safety:
Margin of Safety Study: A combination of fipronil, eprinomectin, praziquantel, and (S)-methoprene was applied topically to 7 to 9 week old healthy kittens at 1, 3, or 5X the maximum dose (8 cats/group) six times at 28 day intervals. One 5X kitten exhibited ataxia, disorientation, and lethargy for 12 hours and exhibited pupil dilation for 24 hours following the 3rd treatment. This 5X kitten exhibited ataxia, disorientation, and lethargy for 6 hours, and moderate pupil dilation for 24 hours following the 4th treatment, and had pupil dilation following the 5th treatment. Hypersalivation was observed for one hour for one 5X kitten following the 1st treatment and one 3X kitten following the 4th treatment. One 5X kitten had slow pupillary light responses for one day after one treatment and one 3X kitten had slow pupillary light responses for 3 hours after one treatment. One control cat had marked pupil dilation and slow pupillary light responses lasting two hours after one treatment. Immediately post-treatment cats in all groups scratched and groomed the application site.
Study in Heartworm Positive Cats: Three groups (0X, 1X and 3X) of 12 young, adult cats, 4.7 to 6.6 months of age, were experimentally infected with adult heartworms (D. immitis) by venous transplantation. All cats were negative for heartworm antibody, antigen and microfilariae prior to transplantation. Two weeks after transplantation, immunoserology verified positive antigen and the presence of microfilaria in all enrolled cats. A combination of fipronil, eprinomectin, praziquantel, and (S)-methoprene was applied topically to cats at 1X or 3X the maximum exposure dose once every 28 days for three consecutive treatments. One cat in the 1X group exhibited cyanotic mucous membranes and tachypnea for 24 hours following the first treatment. The cat recovered and exhibited no abnormal signs following two subsequent treatments. There was no difference between the treatment groups in the number of adult D. immitis recovered at the end of the study.
Oral Administration Study: Oral tolerance was evaluated to assess the effects of accidental oral ingestion. Sixteen cats (8 male and 8 female) ranging in age from 9 - 10 months were studied. Eight cats were orally administered a combination of fipronil, eprinomectin, praziquantel, and (S)-methoprene at 1X the maximum exposure dose; the 8 control cats were sham dosed. All 8 treated cats immediately exhibited hypersalivation after oral administration, and 2 cats vomited and 3 cats were lethargic during the 1-2 hour post-treatment observations. Treated cats continued to hypersalivate and lick lips/mouth for 1-2 hours after oral administration. Cats were monitored for 14 days thereafter, during which one treated cat vomited on Day 12.
Storage
Store at or below 30°C (86°F) with excursions permitted to 40°C (104°F). Protect from light.


