SAVE 15% OFF 15% OFF Use Code EPX15 *

Merck Banamine Transdermal (Flunixin) Pour-On for Beef & Dairy Cattle, 100 mL - [Respiratory Health]
- Description
- Directions
- Reviews
Description
Banamine Transdermal pour-on is indicated for the control of pyrexia associated with bovine respiratory disease and the control of pain associated with foot rot in steers, beef heifers, beef cows, beef bulls intended for slaughter, and replacement dairy heifers under 20 months of age.
Key Benefits
- Used for treatment of "White Muscle Disease" (Selenium-Tocopherol Deficiency).
- Supplied as 100ml bottle.
- Veterinarian's prescription required.
- Approved by FDA
- Each ml of BO-SE® contains the equivalent to 1 mg selenium and 50 mg (68 USP units) vitamin E.
Directions
View Banamine Transdermal Drug Facts Sheet.
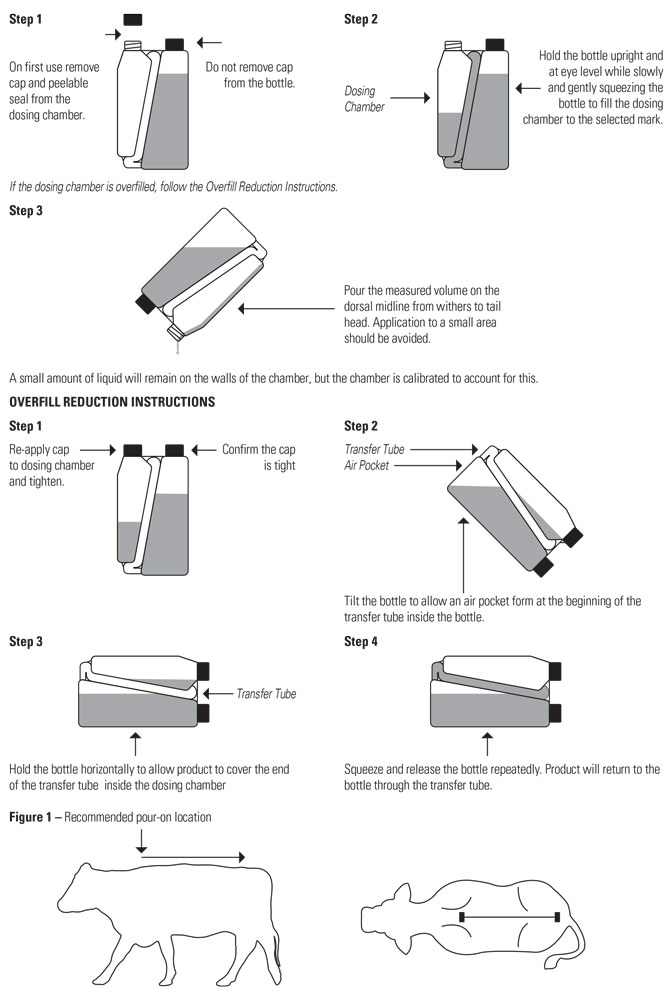
Apply only once at a dose of 3.3 mg flunixin per kg body weight (1.5 mg/lb; 3 mL per 100 lbs) topically in a narrow strip along the dorsal midline from the withers to the tailhead. Round all doses up to the nearest weight increment on the dosing chamber. Do not treat cattle if the hide is wet or may get wet in the six hours after dosing because effectiveness has not been evaluated under wet hide conditions.
Practice the Administration and Overfill Reduction Instructions a few times to become familiar with operating the package before dosing animals.
Contraindications:
NSAIDs inhibit production of prostaglandins which are important in signaling the initiation of parturition. The use of flunixin can delay parturition and prolong labor which may increase the risk of stillbirth. Do not use Banamine Transdermal pour-on within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine.
User Safety Warnings:
Not for use in humans. Keep out of reach of children. Flunixin transdermal solution is a potent non-steroidal anti-inflammatory drug (NSAID), and ingestion may cause gastrointestinal irritation and bleeding, kidney, and central nervous system effects.
This product has been shown to cause severe and potentially irreversible eye damage (conjunctivitis, iritis, and corneal opacity) and irritation to skin in laboratory animals. Users should wear suitable eye protection (face shields, safety glasses, or goggles) to prevent eye contact; and chemical-resistant gloves and appropriate clothing (such as long-sleeve shirt and pants) to prevent skin contact and/or drug absorption. Wash hands after use.
In case of accidental eye contact, flush eyes immediately with water and seek medical attention. If wearing contact lenses, flush eyes immediately with water before removing lenses. In case of accidental skin contact and/or clothing contamination, wash skin thoroughly with soap and water and launder clothing with detergent. In case of ingestion do not induce vomiting and seek medical attention immediately. Probable mucosal damage may contraindicate the use of gastric lavage. Provide product label and/or package insert to medical personnel.
Residue Warnings:
Cattle must not be slaughtered for human consumption within 8 days of the last treatment. Not for use in female dairy cattle 20 months of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in suckling beef calves, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
Precautions:
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction. Banamine transdermal should be used with caution in animals with suspected pre-existing gastric erosions or ulcerations. Concurrent administration of other NSAIDs, corticosteroids, or potentially nephrotoxic drugs should be avoided or used only with careful monitoring because of the potential increase of adverse events.
NSAIDs are known to have potential effects on both parturition (see Contraindications) and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. NSAIDs are known to have the potential to delay parturition through a tocolytic effect. The use of NSAIDs in the immediate postpartum period may interfere with uterine involution and expulsion of fetal membranes. Cows should be monitored carefully for placental retention and metritis if Banamine Transdermal pour-on is used within 24 hours after parturition.
Not for use in dairy or beef bulls intended for breeding because reproductive safety has not been evaluated.


