SAVE 15% OFF 15% OFF Use Code EPX15 *
Atopica for Cats 100mg/ml 5ml - [Skin Allergy Support]
- Notice
- Description
- Ingredients
- Directions
- FAQ
- Reviews
Notices
ON SALE! For a limited time. Add to cart to see automatic coupon. We have the best price and service on the internet.
Description
Atopica for Cats is an oral solution used to control feline allergic dermatitis (allergic skin disease) as seen as broken skin, including facial and neck (miliary dermatitis, eosinophilic plaques, and self-induced alopecia). Signs of allergic skin disease include itching, chewing, licking, skin lesions and hair loss. Some cats may develop lesions that may even appear on the face and neck.
Key Benefits
- Controls feline allergic dermatitis
- Significantly reduces itching and skin lesions
- Comes in an easy-to-administer liquid formulation and includes oral syringe
- Most cats find the taste acceptable, but can be mixed with food
How It Works
Atopica for Cats is an oral solution that works by calming and soothing allergic skin conditions such as feline allergic dermatitis. It contains cyclosporine which stops the itching by blocking white blood cells from organizing and responding to infection. The oral solution is usually very well tolerated by cats and becomes easily absorbed in the intestinal tract.
Indications
ATOPICA for Cats is indicated for the control of feline allergic dermatitis as manifested by excoriations (including facial and neck), miliary dermatitis, eosinophilic plaques, and self-induced alopecia in cats at least 6 months of age and at least 3 lbs (1.4 kg) in body weight.
Ingredients
Active Ingredients:
Cyclosporine
Directions
View Atopica Drug Facts Sheet.
Always provide the Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats and the Information for Cat Owners with prescription.
The initial dose of ATOPICA for Cats is 3.2 mg/lb/day (7 mg/kg/day) as a single daily dose for a minimum of 4 to 6 weeks or until resolution of clinical signs. Following this initial daily treatment period, the dose of ATOPICA for Cats may be tapered by decreasing the frequency of dosing to every other day or twice weekly to maintain the desired therapeutic effect. ATOPICA for Cats should be administered directly on a small amount of food or orally just after feeding. Whenever possible, ATOPICA for Cats should be administered on a consistent schedule with regard to meals and time of day. If a dose is missed, the next dose should be administered (without doubling) as soon as possible, but dosing should be no more frequent than once daily.
The dispensing system includes an oral dosing syringe graduated in 1 lb increments. To dose the cat, the syringe should be filled to the nearest 1 lb corresponding to the cat's body weight (round down if 0.1 to 0.4 lb, round up if 0.5 to 0.9 lb).
Each pound graduation on the syringe delivers a volume of 0.032 mL providing 3.2 mg/lb. Do not rinse or clean the oral dosing syringe between uses. (See Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats)
Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats(cyclosporine oral solution) USP MODIFIED
Assembling the Dispensing System
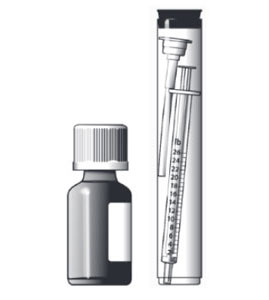 |
The dispensing system consists of 4 parts:
|
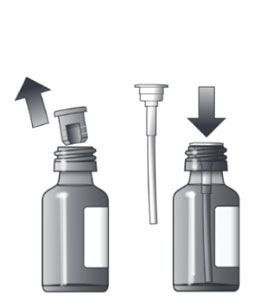 |
Fitting the Plastic Adapter into the New Bottle of Medicine
Note: To prepare a dose, carefully follow the instructions for Preparing a Dose of Medicine. |
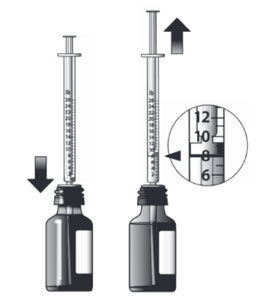 |
Preparing a Dose of Medicine
You can now place the oral dosing syringe over a small amount of food or introduce the syringe in the mouth of your cat and push the medicine out of the syringe. See Information for Cat Owners for complete administration instructions. Do not rinse or clean the oral dosing syringe between uses. Store the oral dosing syringe in the plastic tube between each use. ATOPICA for Cat's should be stored in the original container at room temperature between 59 and 77°F. Once opened, use contents within two months for the 5 mL container and 11 weeks for the 17 mL container. Close the bottle with the child-resistant screw cap after use. Keep out of reach of children! |
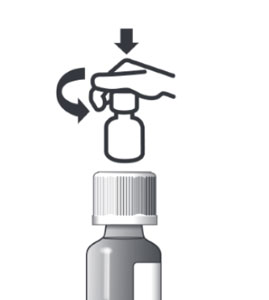 |
Always close the bottle with the child-resistant screw cap after use. To provide a child-resistant closure, push down on the child-resistant screw cap as you turn it. |
Caution:
Federal (USA) Law restricts this drug to use by or on the order of a licensed veterinarian.
Contraindications:
- Do not use in cats with a history of malignant disorders or suspected malignancy.
- Do not use in cats infected with feline leukemia virus (FeLV) or feline immunod eficiency virus (FIV).
- Do not use in cats with a hypersensitivity to cyclosporine.
Warnings:
ATOPICA for Cats is a systemic immunosuppressant that may increase the susceptibility to infection and the development of neoplasia. One of 205 field study cats died of the effusive form of feline infectious peritonitis. (See Adverse Reactions) Persistent, progressive weight loss that resulted in hepatic lipidosis occurred in 2 of 205 cats on treatment with ATOPICA for Cats in field studies. Monitoring of body weight is recommended. (See Adverse Reactions)
Human Warnings:
Not for human use. Keep this and all drugs out of reach of children. For use only in cats.
Special precautions to be taken when administering ATOPICA for Cats:
Do not eat, drink, smoke, or use smokeless tobacco while handling ATOPICA for Cats.Wash hands after administration.
In case of accidental ingestion, seek medical advice immediately and provide the package insert or the label to the physician.
People with known hypersensitivity to cyclosporine should avoid contact with ATOPICA for Cats.
Precautions:
The safety and effectiveness of ATOPICA for Cats has not been established in cats less than 6 months of age or less than 3 lbs (1.4 kg) body weight.
ATOPICA for Cats is not for use in breeding cats, pregnant or lactating queens.Cats should be tested and found to be negative for FeLV and FIV infections before treatment.As with any immunosuppressive regimen, exacerbation of sub-clinical neoplastic and infectious conditions may occur. ATOPICA for Cats is not for use with other immunosuppressive agents.
Cats that are seronegative for Toxoplasma gondii may be at risk of developing clinical toxoplasmosis if they become infected while under treatment, which can be fatal. In a controlled laboratory study, cats seronegative for T. gondii were administered cyclosporine and subsequently infected with T. gondii, resulting in increased susceptibility to infection and subsequent expression of toxoplasmosis. Cyclosporine did not increase T. gondii oocyst shedding (see Animal Safety). Potential exposure of seronegative cats to T. gondii should be avoided (e.g. keep indoors, avoid raw meat or scavenging).
In cases of clinical toxoplasmosis or other serious systemic illness, stop treatment with cyclosporine and initiate appropriate therapy.
ATOPICA for Cats may cause elevated levels of serum glucose, creatinine, and urea nitrogen. ATOPICA for Cats should be used with caution in cases with diabetes mellitus or renal insuffciency.
ATOPICA for Cats should be used with caution with drugs that affect the P-450 enzyme system. Simultaneous administration of ATOPICA for Cats with drugs that suppress the P-450 enzyme system, such as azoles (e.g. ketoconazole), may lead to increased plasma levels of cyclosporine.
Treatment with ATOPICA for Cats may result in decreased immune response to vaccination. Naïve cats may not develop protective titers during treatment (see Animal Safety).
Informaion for Cat Owners:
Owners should be advised to discontinue ATOPICA for Cats and contact their veterinarian in case of signs of serious illness and/or persistent, progressive weight loss. Owners should be informed of the risks of increased susceptibility to infection and the development of neoplasia, and they should be provided advice on how to avoid exposure of their cat to Toxoplasma gondii infection.
ATOPICA for Cats is indicated for the control of feline allergic dermatitis. Cats with allergic dermatitis scratch, lick and chew their skin which can cause red, raised crusty bumps, open sores and/or hair loss.
Allergic dermatitis is a common skin disease in cats and is caused by allergens such as house dust mites or pollens which stimulate an exaggerated immune response. The disease is chronic, recurrent, and requires lifelong management.
This summary contains important information about ATOPICA for Cats. You should read this information before starting your cat on ATOPICA for Cats. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want to know more about ATOPICA for Cats.
Storage:
ATOPICA for Cats should only be dispensed in the original container and stored at controlled room temperature between 59 and 77°F (15-25°C). Once opened, use contents within two months for the 5 mL container and 11 weeks for the 17 mL container.
FAQ
ATOPICA for Cats should be given daily until improvement is seen. This will generally be the case within 4-6 weeks. You should contact your veterinarian if you are not satisfied with your cat's response. Once the signs of allergic dermatitis are satisfactorily controlled, your veterinarian may reduce the frequency of administration of the product. Dose adjustment should only be carried out in consultation with your veterinarian.
Your veterinarian will perform a clinical assessment at regular intervals and adjust the frequency of administration up or down according to the clinical response obtained.
Your cat should not be given ATOPICA for Cats if s/he:
- Has a history of cancer or may possibly have cancer currently
- Has been diagnosed with feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV)
- Is hypersensitive to cyclosporine
Tell your veterinarian about:
- Any digestive upset (vomiting or diarrhea) your cat has had
- Any history of lack of appetite and/or weight loss your cat has had
- Any serious disease or health conditions your cat has had
- Any allergies that your cat has now or has had
- All medications that you are giving your cat or plan to give your cat, including those you can get without prescription (over the counter) and any dietary supplements
- If you plan to breed your cat, or if your cat is pregnant or nursing
Talk to your veterinarian about:
- hat tests might be done before ATOPICA for Cats is prescribed
- The potential side effects your cat may experience while taking ATOPICA for Cats
- How often your cat may need to be examined by your veterinarian
- The risks and benefits of using ATOPICA for Cats
ATOPICA for Cats, like all other drugs, may cause some side effects in individual cats. These are normally mild, but serious side effects have been reported in cats taking ATOPICA for Cats. Serious side effects can, in rare situations, result in death. It is important to stop the medication and contact your veterinarian immediately if you think your cat may have a medical problem or side effect while on ATOPICA for Cats. To report adverse effects, access medical information, or obtain additional product information call 1-888-545-5973.
In clinical studies, the most commonly reported side effect was vomiting. In most cases, the vomiting stopped with continued use. Weight loss, diarrhea, decreased appetite, lethargy, and drooling were the next most frequent side effects observed.
Persistent, progressive weight loss may be associated with more serious side effects. You should monitor your cat's appetite and body weight. If you think that your cat is losing weight, you should contact your veterinarian.
ATOPICA for Cats may increase susceptibility to infection and to the development of tumors.
Do not eat, drink, smoke, or use smokeless tobacco while handling ATOPICA for Cats.
Wash hands after administration.In case of accidental ingestion, seek medical advice immediately and show the package insert or the label to the physician.
People with known hypersensitivity to cyclosporine should avoid contact with ATOPICA for Cats.
TOPICA for Cats should not be given with other drugs that may lower the immune response.
ATOPICA for Cats has been safely used in conjunction with other common medications. However, interactions with certain medications are possible. Therefore, always tell your veterinarian about all medications that you have given your cat in the past and all medications that you are planning to give with ATOPICA for Cats.
Toxoplasma gondii is a protozoal parasite that cats can become infected with from eating raw meat. Infection may lead to serious illness (clinical toxoplasmosis). Cats that have not been exposed to Toxoplasma gondii may be at risk of developing clinical toxoplasmosis if they become infected while under treatment with ATOPICA for Cats, which can be fatal. To avoid infection keep your cat indoors, do not feed raw meat, and do not allow your cat to hunt.
If your cat becomes seriously ill, consult your veterinarian who will recommend the appropriate treatment.
This sheet provides a summary of information about ATOPICA for Cats. If you have any questions or concerns about ATOPICA for Cats or allergic dermatitis in cats, talk to your veterinarian.
As with all prescribed medications, ATOPICA for Cats should only be given to the cat for which it was prescribed. It should be given to your cat only for the condition for which it was prescribed, at the prescribed dose, and as directed by your veterinarian.







