SAVE 15% OFF 15% OFF Use Code EPX15 *
Adequan for Dogs - Canine Injection (5 ml) - [Inflammation Relief]
- Notice
- Description
- Ingredients
- Directions
- FAQ
- Reviews
Notices
This is our Rock-Bottom-Price! Sorry, Promotional offers do not apply (See Exclusions.)
Description
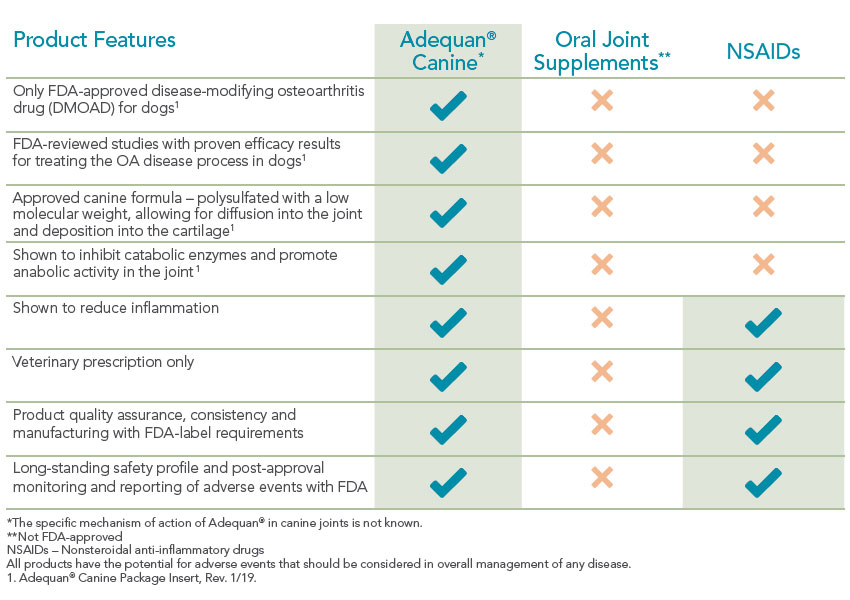
Adequan Canine (polysulfated glycosaminoglycan) is the only FDA-approved disease-modifying osteoarthritis drug (DMOAD) for dogs. Unlike supplements or NSAIDs that simply mask symptoms, Adequan Canine works at the source to help protect and preserve joint cartilage.1
It’s designed to inhibit cartilage loss and may help your dog feel more mobile, active, and comfortable by:
Key Benefits
- Restoring healthy joint lubrication
- Relieving inflammation that contributes to joint pain
- Rebuilding the foundational components of cartilage
Adequan Canine is more than a symptom reliever—it's a proactive treatment that targets the disease itself. With intramuscular injections, this treatment helps slow the progression of canine osteoarthritis and supports joint function from the inside out.1
While the precise mechanism of action in canine joints has not been fully defined, Adequan Canine is clinically shown to support joint health and slow the progression of osteoarthritis.1
How It Works
Adequan binds to cartilage matrix components to help prevent further joint degradation. Its effects include:
- Reducing joint inflammation and associated pain
- Inhibiting enzymes and inflammatory compounds that damage cartilage
- Stimulating cartilage repair and improving synovial fluid quality for better joint movement

Indications
Adequan Canine is indicated for intramuscular use in dogs to control the signs associated with non-infectious degenerative and/or traumatic arthritis of synovial joints.
Ingredients
| Active Ingredients | Amount |
|---|---|
| Polysulfated Glycosaminoglycan | 100 mg |
| Benzyl alcohol (a preservative) | 0.9% v/v |
| Water | |
Other Ingredients:
Sodium hydroxide and/or hydrochloric acid added when necessary to adjust pH. Sodium Chloride may be added to adjust tonicity.
Directions
View Adequan Drug Facts Sheet.
Practice aseptic techniques in withdrawing each dose todecrease the possibility of post-injection bacterial infections. Adequately clean anddisinfect the stopper prior to entry with a sterile needle and syringe. Use only sterileneedles, and use each needle only once.
The vial stopper may be punctured a maximum of 10 times.
The recommended dose of Adequan® Canine is 2 mg/lb body weight (.02 mL/lb, or 1 mL per50 lb), by intramuscular injection only, twice weekly for up to 4 weeks (maximum of 8 injections). Do not exceed the recommended dose or therapeutic regimen. Do not mix Adequan® Canine withother drugs or solvents.
How Adequan® Canine (polysulfated glycosaminoglycan) can help extend a dog's mobility over a lifetime.
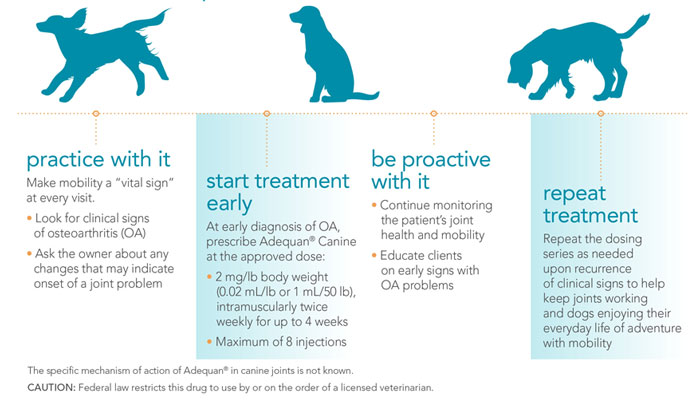
The FDA-approved formula that's never been duplicated.
- Helps control signs associated with non-infectious degenerative and/or traumatic arthritis of canine synovial joints.1
- Enters the joints quickly, within 2 hours, to help control signs of arthritis.1
- Therapeutic concentrations in synovial fluid and articular cartilage last up to 3 days.1
What does Adequan® Canine have to offer?
Important Safety Information
Adequan® Canine should not be used in dogs who are hypersensitive to PSGAG or who have a known or suspected bleeding disorder. It should be used with caution in dogs with renal or hepatic impairment. Adverse reactions in clinical studies (transient pain at injection site, transient diarrhea, and abnormal bleeding) were mild and self-limiting. In post approval experience, death has been reported in some cases; vomiting, anorexia, depression/lethargy and diarrhea have also been reported. The safe use of PSGAG in breeding, pregnant or lactating dogs has not been evaluated.
Tips to keep dog's joints healthy at every stage of life.
- Keep dog at a healthy weight
- Encourage regular movement and exercise
- Provide balanced nutrition, particularly during growth phase
- Understand the early signs of a potential joint problem
- Pet parents and veterinarians work collaboratively to identify a problem early and establish a treatment plan
- Comply with veterinarian recommendations
Why treat just the signs of canine osteoarthritis when you can proactively treat the disease?
Special diets, supplements and anti-inflammatory drugs can play a part in managing joint disease but none of them specifically address the underlying cartilage deterioration. Once cartilage wears away completely, it can't be restored so it's vital to help maintain its use.1 That's what makes Adequan® Canine (polysulfated glycosaminoglycan) different. It empowers you to proactively treat the disease and not just the signs of canine osteoarthritis.2
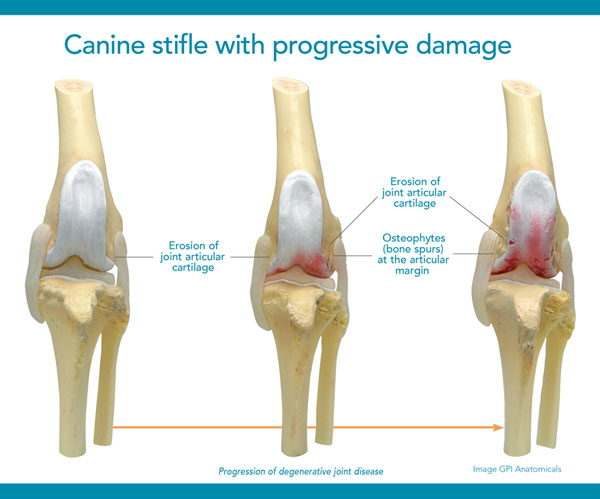
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Storage
Store at 20° to 25°C (68° to 77°F) excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature). Avoid prolonged exposure totemperatures ≥40°C (104°F).
Use within 28 days of first puncture and puncture a maximum of 10 times. Dispose of spentneedles in accordance with all federal, state and local environmental laws.
Contraindications
Do not use in dogs showing hypersensitivity to PSGAG. PSGAG is asynthetic heparinoid; do not use in dogs with known or suspected bleeding disorders.
Precautions:
The safe use of Adequan® Canine used in breeding, pregnant, or lactating dogshas not been evaluated. Use with caution in dogs with renal or hepatic impairment.
Adverse Reactions:
In the clinical efficacy trial, 24 dogs were treated with Adequan®Caninetwice weekly for 4 weeks. Possible adverse reactions were reported after 2.1% of the injections.These included transient pain at the injection site (1 incident), transient diarrhea (1 incident eachin 2 dogs), and abnormal bleeding (1 incident). These effects were mild and self-limiting and didnot require interruption of therapy.
Post Approval Experience (2014)
The following adverse events are based on voluntary, post-approval reporting. Not all adversereactions are reported to FDA/CVM. It is not always possible to reliably estimate the adverseevent frequency or establish a causal relationship to product exposure using these data. Thesigns reported are listed in decreasing order of reporting frequency.
- Vomiting
- Anorexia
- Depression/lethargy
- Diarrhea.
In some cases, death has been reported
To report suspected adverse drug events, contact Luitpold Pharmaceuticals, Inc. at 1-800- 458-0163. For additional information about adverse drug experience reporting for animaldrugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Warnings:
Not for use in humans. Keep this and all medications out of reach of children.
Efficacy:
Efficacy of Adequan® Canine was demonstrated in two studies. A laboratory study usingradiolabeled PSGAG established distribution of PSGAG into canine serum and synovial fluidfollowing a single intramuscular injection of 2 mg/lb. A clinical field trial was conducted in dogsdiagnosed with radiographically-confirmed traumatic and/or degenerative joint disease of 1 or 2 joints. Joints evaluated included hips, stifles, shoulders, hocks and elbows. Fifty-one dogs wererandomly assigned to receive either Adequan® Canine at 2 mg/lb of body weight or 0.9% saline.
Both treatments were administered by intramuscular injection twice weekly for 4 weeks (8 injections total). Investigators administering treatment and evaluating the dogs were unaware ofthe treatment assignment. A total of 71 limbs in 51 dogs were evaluated. Of these, 35 limbs in 24 dogs were in the Adequan® Canine treated group. Each lame limb was scored for lameness ata walk, lameness at a trot, pain, range of motion, and functional disability. The scores for theindividual parameters were combined to determine a total orthopedic score. At the end of thetreatment period, dogs treated with Adequan®Canine showed a statistically significantimprovement in range of motion and total orthopedic score over placebo treated control dogs.
Toxicity:
In a subacute toxicity study, 32 adult beagle dogs (4 males and 4 females per treatmentgroup) received either 0.9% saline solution or PSGAG at a dose of 5 mg, 15 mg, or 50 mg per kgof body weight (approximately 2.3, 6.8, or 22.7 mg/lb), via intramuscular injection twice weekly for13 weeks. PSGAG doses represent approximately 1X, 3X, and 10X the recommended dosage of2 mg/lb, and more than 3 times the recommended 4-week duration of treatment. Necropsies wereperformed 24 hours after the final treatment. During week 12, one dog in the 50 mg/kg dosagegroup developed a large hematoma at the injection site which necessitated euthanasia. No othermortalities occurred during the treatment period. Statistically significant changes in the 50 mg/kggroup included increased prothrombin time, reduced platelet count, an increase in ALT andcholesterol, and increased liver and kidney weights. Increased cholesterol and kidney weightswere also noted in the 15 mg/kg group. Microscopic lesions were noted in the liver (Kupffer cellscontaining eosinophilic foamy cytoplasm), kidneys (swollen, foamy cells in the proximalconvoluted tubules), and lymph nodes (macrophages with eosinophilic foamy cytoplasm) in the15 mg/kg and 50 mg/kg groups. Intramuscular inflammation, hemorrhage, and degeneration wereseen in all 3 PSGAG treated groups; the incidence and severity appeared dose related.
Pharmacology:
The specific mechanism of action of Adequan®in canine joints is not known.PSGAG is characterized as a “disease modifying osteoarthritis drug”. Experiments conducted invitrohave shown PSGAG to inhibit certain catabolic enzymes which have increased activity ininflamed joints, and to enhance the activity of some anabolic enzymes. For example, PSGAG hasbeen shown to significantly inhibit serine proteinases. Serine proteinases have beendemonstrated to play a role in the Interleukin-l mediated degradation of cartilage proteoglycansand collagen. PSGAG is reported to be an inhibitor of Prostaglandin E2 (PGE2) synthesis. PGE2has been shown to increase the loss of proteoglycan from cartilage. PSGAG has been reported toinhibit some catabolic enzymes such as elastase, stromelysin, metalloproteases, cathepsin B1,and hyaluronidases, which degrade collagen, proteoglycans, and hyaluronic acid in degenerativejoint disease. Anabolic effects studied include ability to stimulate the synthesis of protein, collagen,proteoglycans, and hyaluronic acid in various cells and tissues invitro. Cultured human and rabbitchondrocytes have shown increased synthesis of proteoglycan and hyaluronic acid in thepresence of PSGAG. PSGAGs have shown a specific potentiating effect on hyaluronic acidsynthesis by synovial membrane cells in vitro.
Absorption, distribution, metabolism, and excretion of PSGAG following intramuscular injectionhave been studied in several species, including rats, rabbits, humans, horses and dogs.
Studies in rabbits showed maximum blood concentrations of PSGAG following IM injection werereached between 20 to 40 minutes following injection, and that the drug was distributed to alltissues studied, including articular cartilage, synovial fluid, adrenals, thyroid, peritoneal fluid,lungs, eyes, spinal cord, kidneys, brain, liver, spleen, bone marrow, skin, and heart.
Following intramuscular injection of PSGAG in humans, the drug was found to be bound to serumproteins. PSGAG binds to both albumin and chi and beta-globulins and the extent of the bindingis suggested to be 30 to 40%. Therefore, the drug may be present in both bound and free form inthe bloodstream. Because of its relatively low molecular weight, the synovial membrane is not asignificant barrier to distribution of PSGAG from the bloodstream to the synovial fluid. Distributionfrom the synovial fluid to the cartilage takes place by diffusion. In the articular cartilage the drug isdeposited into the cartilage matrix.
Serum and synovial fluid distribution curves of PSGAG have been studied in dogs and appearsimilar to those found in humans and rabbits.
In rabbits, metabolism of PSGAG is reported to take place in the liver, spleen, and bone marrow.Metabolism may also occur in the kidneys. PSGAG administered intramuscularly and not proteinbound or bound to other tissues is excreted primarily via the kidneys, with a small proportionexcreted in the feces.
FAQ
Reviews
- It really works and my dog is almost pain free after 3 shots.
- There is not any Con about this product
- See review
- Easy to administer, intermuscular or sq.
- 100 lb goat has more issues with injection than 50 lb dog!
- Pricey, but worth it.
- Economical and efficient
- Fast acting. No side effects for our dog. Easy to give sub cutaneous injections. Very cost effective role over the price we paid through our vet.
- None. Absolutely none.
- Easy to use. Simple directions.
- Forgot to also order syringes - maybe they can put an * to help remind us......












