SAVE 17% OFF 17% OFF Use Code LUCKY17 *
Vetsulin for Dogs & Cats - Insulin U-40 10ml - [Diabetic Injection]
- Notice
- Description
- Ingredients
- Directions
- FAQ
- Reviews
Notices
This item will ship via Express 1-2 day mail with a flat rate fee of $36.95 and will be packed in an insulated box with ice packs. Items that are required to ship this method will be listed in the item description. Orders placed after 2:00 PM ET (11:00 AM PT) may not end up being able to ship out the same day.
Restrictions: All foreign orders are ineligible. Free shipping is ineligible.
Note: Vaccine orders are only shipped out on Mon-Wed. due to Express service only being able to deliver during weekdays. We cannot be responsible for vaccine or other refrigerated orders that are delayed because no one is present at the time it is delivered.
Description
Vetsulin is the first and only FDA-approved insulin specifically formulated for the treatment of diabetes mellitus in both dogs and cats. This porcine insulin zinc suspension helps regulate blood glucose levels, reducing the clinical signs of diabetes such as excessive thirst, urination, and weight loss. Vetsulin is available in a 10 mL vial and should be administered using U-40 insulin syringes (sold separately). This product requires a prescription from a licensed veterinarian and must be kept refrigerated at all times.
Key Benefits
- Effectively controls blood glucose levels in diabetic dogs and cats
- Helps alleviate common symptoms of diabetes, including excessive thirst and urination
- Noticeable improvement is often seen within just a few days of starting treatment
- Only FDA-approved insulin for both dogs and cats in the United States
How It Works
Insulin is a hormone produced by the pancreas that helps regulate blood sugar. In diabetic pets, the body either doesn't produce enough insulin or cannot effectively use it. Vetsulin contains porcine insulin, which is structurally similar to canine insulin, allowing for effective absorption and glucose regulation. It works by lowering blood sugar levels and managing the symptoms associated with diabetes mellitus.
Indications
Vetsulin is indicated for the management of hyperglycemia and the associated clinical signs in dogs and cats diagnosed with diabetes mellitus.
Important Note
Vetsulin must be stored in the refrigerator between 36°F and 46°F (2°C and 8°C). Do not freeze. Use only with U-40 syringes. Always follow your veterinarian’s dosage instructions carefully and monitor your pet regularly.
Ingredients
Active Ingredient
- Porcine Insulin Zinc Suspension: A purified insulin derived from pigs, formulated with zinc crystals to provide intermediate-acting blood glucose control in diabetic dogs and cats.
Inactive Ingredients
- Zinc (as Zinc Chloride): Stabilizes the insulin and extends its duration of action.
- Buffering Agents: Help maintain optimal pH for effectiveness and stability.
- Preservatives: Includes methylparaben and propylparaben to ensure sterility and shelf life.
- Glycerol and Water: Used as the aqueous suspension medium for uniform insulin delivery.
Directions
View Vetsulin Drug Facts Sheet.
Dosage and Administration
FOR SUBCUTANEOUS INJECTION IN DOGS AND CATS ONLY
Vials: USE OF A SYRINGE OTHER THAN A U-40 SYRINGE WILL RESULT IN INCORRECT DOSING.
Shake the vial thoroughly until a homogeneous, uniformly milky suspension is obtained. Foam on the surface of the suspension formed during shaking should be allowed to disperse before the product is used and, if required, the product should be gently mixed to maintain a homogeneous, uniformly milky suspension before use. Clumps or white particles can form in insulin suspensions: do not use the product if visible clumps or white particles persist after shaking thoroughly.
Cartridges: VETSULIN CARTRIDGES SHOULD BE USED EXCLUSIVELY WITH VETPEN AND 29G/12 MM PEN NEEDLES.
Prior to loading vetsulin cartridges, shake the cartridge until a homogeneous, uniformly milky suspension is obtained. Clumps or white particles can form in insulin suspensions: do not use the product if visible clumps or white particles persist after shaking.
The detailed instructions for use provided with VetPen should be strictly followed.
The injection should be administered subcutaneously, 2 to 5 cm (3/4 to 2 in) from the dorsal midline, varying from behind the scapulae to the mid-lumbar region and alternating sides.
Always provide the Owner Information Sheet with each prescription.
Dogs
The initial recommended vetsulin dose is 0.5 IU insulin/kg body weight. Initially, this dose should be given once daily concurrently with, or right after a meal.
Twice daily therapy should be initiated if the duration of insulin action is determined to be inadequate. If twice daily treatment is initiated, the two doses should each be 25% less than the once daily dose required to attain an acceptable nadir. For example, if a dog receiving 20 units of vetsulin® once daily has an acceptable nadir but inadequate duration of activity, the vetsulin®dose should be changed to 15 units twice daily.
The veterinarian should re-evaluate the dog at appropriate intervals and adjust the dose based on clinical signs, urinalysis results, and glucose curve values until adequate glycemic control has been attained. Further adjustments in dosage may be necessary with changes in the dog's diet, body weight, or concomitant medication, or if the dog develops concurrent infection, inflammation, neoplasia, or an additional endocrine or other medical disorder.
Cats
The initial recommended dose in cats is 1 to 2 IU per injection. The injections should be given twice daily at approximately 12 hour intervals. For cats fed twice daily, the injections should be given concurrently with, or right after each meal. For cats fed ad libitum, no change in feeding schedule is needed
The veterinarian should re-evaluate the cat at appropriate intervals and adjust the dose based on clinical signs, urinalysis results, and glucose curve values until adequate glycemic control has been attained. Further adjustments in dosage may be necessary with changes in the cat's diet, body weight, or concomitant medication, or if the cat develops concurrent infection, inflammation, neoplasia, or an additional endocrine or other medical disorder.
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Contraindications:
Dogs and cats known to have a systemic allergy to pork or pork products should not be treated with vetsulin. vetsulin® is contraindicated during periods of hypoglycemia.
Warnings:
User Safety: For use in animals only. Keep out of the reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. Accidental injection may cause clinical hypoglycemia. In case of accidental injection, seek medical attention immediately. Exposure to product may induce a local or systemic allergic reaction in sensitized individuals.
Animal Safety: Owners should be advised to observe for signs of hypoglycemia (see Owner Information Sheet). Use of this product, even at established doses, has been associated with hypoglycemia. An animal with signs of hypoglycemia should be treated immediately. Glucose should be given orally or intravenously as dictated by clinical signs. Insulin should be temporarily withheld and, subsequently, the dosage should be adjusted, if indicated. Any change in insulin should be made cautiously and only under a veterinarian's supervision. Changes in insulin strength, manufacturer, type, species (animal, human) or method of manufacture (rDNA versus animal-source insulin) may result in the need for a change in dosage.
Appropriate diagnostic tests should be performed to rule out endocrinopathies in pets that are difficult to regulate (e.g., hyperadrenocorticism in dogs and hyperthyroidism in cats).
Precautions:
Animals presenting with severe ketoacidosis, anorexia, lethargy, and/or vomiting should be stabilized with short-acting insulin and appropriate supportive therapy until their condition is stabilized. As with all insulin products, careful patient monitoring for hypoglycemia and hyperglycemia are essential to attain and maintain adequate glycemic control and prevent associated complications. Overdosage can result in profound hypoglycemia and death. Progestogens, certain endocrinopathies, and glucocorticoids can have an antagonistic effect on insulin activity. Intact bitches should be ovariohysterectomized.
Progestogen and glucocorticoid use should be avoided.

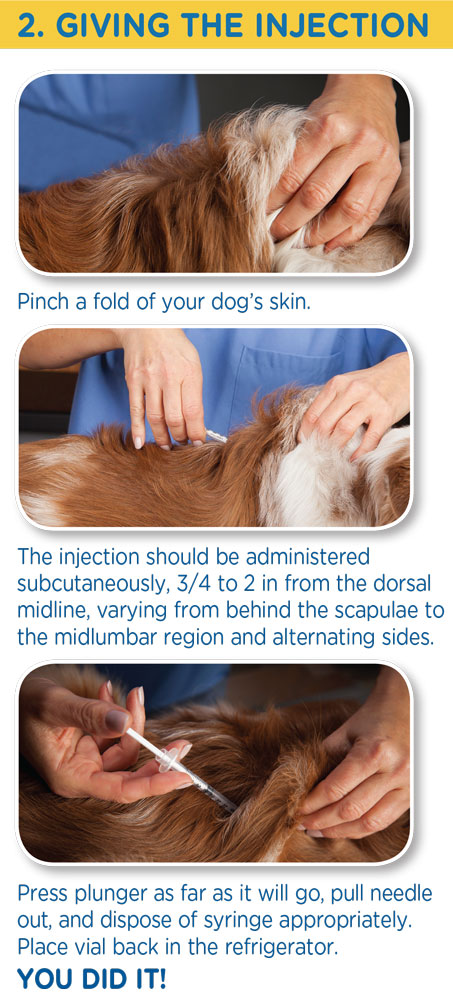
Injection Tips
- If necessary, ask someone to hold your dog prior to injecting Vetsulin (porcine insulin zinc suspension).
- Location of injection should be altered from behind shoulder blade to slightly in front of hip bone - it depends upon your veterinarian's recommendation and what suits you and your dog.
- Alternate injection site between left and right side for more comfort.
- Give your dog her favorite food as you administer the injection.
FAQ
Vetsulin facilitates a more optimal treatment protocol than current human insulin products.
- Where biosynthetic human insulin is only available in 100 IU/mL concentrations, Vetsulin has a 40 IU/mL concentration allowing for more accurate dosing of small animals and reducing the risk of under- or overdosing.
- Vetsulin is administered with U-40 insulin syringe or VetPen, making it easier for the client to read and deliver the dose.
- The duration of activity may be longer.
Reviews
- Great packing
- Expensive
- Excellent communication by email and phone. Shipping on time
- Sometimes I get emails that say I need to contact my vet when my auto ship is about to send and I know I don
- Prices
- Fedex
- Works to control diabetes in my dog
- Price























