- Moxidectin provides monthly protection from heartworm disease.
- Sarolaner treats & controls tick infestations, and treats & prevents flea infestations.
- Pyrantel treats & controls roundworms and hookworms.
SAVE 17% OFF 17% OFF Use Code LUCKY17 *
Simparica Trio for Dogs - 44.1-88 lbs (1 Chewable Tablet) - [Flea, Ticks & Heartworm]
- Notice
- Description
- Ingredients
- Directions
- FAQ
- Reviews
Notices
SIMPARICA TRIO ON SALE! Discounted price can be seen in cart.
Description
Simparica Trio is a chewable tablet for dogs that provides broad-spectrum protection against fleas, ticks, heartworms, roundworms, hookworms, and whipworms. It contains three active ingredients - sarolaner, moxidectin, and pyrantel - that work together to protect your dog against these parasites. This chewable tablet is easy to administer orally, making it convenient for pet owners. Simparica Trio is a monthly treatment that provides protection for a full month with just one tablet. Please note that Simparica Trio is available only with a prescription from a licensed veterinarian, ensuring that it is used safely and effectively.
Simparica Trio comes in a chewable tablet form, making it convenient to administer to dogs. The medication is typically prescribed by a veterinarian and is administered once a month for ongoing parasite protection.
It's important to note that Simparica Trio is specifically designed for dogs and should not be used on other animals or humans. Additionally, it's always recommended to consult with a veterinarian before starting any new medication to ensure it is appropriate for your dog and to determine the correct dosage based on your dog's size and health condition.
Key Features:
- Broad-Spectrum Protection: Protects against fleas, ticks, heartworms, roundworms, hookworms, and whipworms.
- Chewable Tablet: Easy to administer orally, making it convenient for pet owners.
- Monthly Treatment: Provides protection for a full month with one tablet.
- Suitable for Dogs: Formulated specifically for use in dogs.
- Prescription Only: Available only with a prescription from a licensed veterinarian.
Indications:
Simparica Trio is indicated for the prevention and treatment of several parasitic infections in dogs. Here are the main indications for using Simparica Trio:
- Fleas: Simparica Trio kills fleas (Ctenocephalides felis) and provides at least one month of protection against flea infestations.
- Ticks: Simparica Trio kills four common species of ticks that infest dogs: the black-legged tick (Ixodes scapularis), the American dog tick (Dermacentor variabilis), the Gulf Coast tick (Amblyomma maculatum), and the lone star tick (Amblyomma americanum). It provides at least one month of protection against ticks.
- Heartworm disease: Simparica Trio is effective in preventing heartworm disease (Dirofilaria immitis) by eliminating the infective larvae transmitted by mosquitoes. It should be administered monthly to ensure continuous protection against heartworm infection.
- Intestinal worms: Simparica Trio controls and treats intestinal worm infections in dogs. It is effective against roundworms (Toxocara canis, Toxascaris leonina), hookworms (Ancylostoma caninum, Uncinaria stenocephala), and whipworms (Trichuris vulpis). It helps to eliminate and prevent these parasites from establishing in the dog's intestines.
It's important to note that Simparica Trio is intended for use in dogs only and is available in different strengths based on the weight of the dog. It should be administered as directed by a veterinarian, and the dosing schedule should be followed consistently for optimal effectiveness.
How it works:
Simparica Trio works through the combined action of its three active ingredients: sarolaner, moxidectin, and pyrantel. Let's look at how each ingredient works:
- Sarolaner: Sarolaner is an ectoparasiticide, which means it targets external parasites like fleas and ticks. When a dog ingests Simparica Trio, sarolaner is absorbed into the bloodstream. It then circulates throughout the dog's body. Sarolaner targets the nervous system of fleas and ticks, specifically by binding to receptors called gamma-aminobutyric acid (GABA) and glutamate receptors in these parasites. This binding disrupts the normal functioning of the parasites' nervous system, leading to paralysis and subsequent death of the fleas and ticks.
- Moxidectin: Moxidectin is an anthelmintic agent that is primarily effective against heartworms, but it also has activity against certain intestinal worms. Once absorbed into the bloodstream, moxidectin is distributed throughout the dog's body. It works by binding to specific receptors called glutamate-gated chloride channels in the nervous system of parasites. This binding causes an influx of chloride ions, leading to hyperpolarization and paralysis of the parasites. Moxidectin kills and prevents the development of heartworm larvae and provides control against certain species of roundworms and hookworms.
- Pyrantel: Pyrantel is an anthelmintic agent that primarily targets intestinal worms. After being ingested, pyrantel is poorly absorbed from the dog's gastrointestinal tract, and it remains primarily in the intestinal lumen. Pyrantel works by blocking the parasites' neuromuscular junction, specifically by causing depolarization and paralysis of the worms. This effect makes the worms lose their grip on the intestinal wall, allowing them to be expelled from the dog's system through normal bowel movements.
By combining these three active ingredients, Simparica Trio provides a comprehensive approach to parasite control in dogs. It acts against fleas, ticks, heartworms, and common intestinal worms, offering a broad spectrum of protection. Regular monthly administration of Simparica Trio helps ensure continuous coverage and reduces the risk of parasite infestations and associated diseases in dogs.
Specifications:
- Brand: Simparica Trio
- Active Ingredients: Sarolaner, moxidectin, pyrantel
- Type: Chewable Tablet
- Indicated For: Flea, tick, heartworm, roundworm, and hookworm prevention in dogs
- Dosage Form: Chewable tablet
- Strength: Various strengths available
Ingredients
Directions
Dosage:
Simparica Trio is given orally once a month, at the recommended minimum dose of 0.54 mg/lb (1.2 mg/kg) sarolaner, 0.011 mg/lb (24 mg/kg) moxidectin, and 2.27 mg/lb (5 mg/kg) pyrantel (as pamoate salt).
View Simparica Trio Drug Facts Sheet.
Dosage Table
| Body Weight | Sarolaner per Tablet (mg) | Moxidectin per Tablet (mg) | Pyrantel per Tablet (mg) | Number of Tablets Administered | |
|---|---|---|---|---|---|
| 2.8 to 5.5 lbs. | 3 | 0.06 | 12.5 | One (Yellow) | |
| 5.6 to 11.0 lbs. | 6 | 0.12 | 25 | One (Purple) | |
| 11.1 to 22.0 lbs. | 12 | 0.24 | 50 | One (Blue) | |
| 22.1 to 44.0 lbs. | 24 | 0.100 | 50 | One (Blue) | |
| 44.1 to 88.0 lbs. | 48 | 0.96 | 200 | One (Green) | |
| 88.1 to 132.0 lbs. | 72 | 1.44 | 300 | One (Brown) | |
| >132.1 lbs. | Administer the appropriate combination of tablets | ||||

Administration:
Simparica Trio can be offered by hand, in the food, or administered like other tablet medications. Care should be taken that the dog consumes the complete dose, and treated animals should be observed for a few minutes to ensure that part of the dose is not lost or refused. If a dose is missed, administer Simparica Trio and resume a monthly dosing schedule.
Simparica Trio should be administered at monthly intervals.
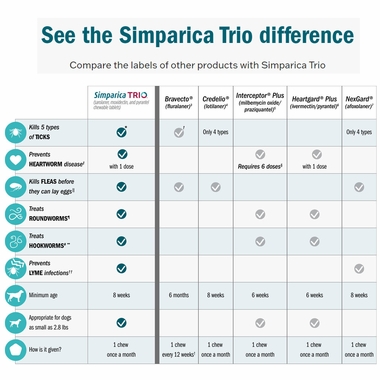
See the Simparica Trio differenceCompare the labels of other products with Simparica Trio

Heartworm Prevention:
Simparica Trio should be administered at monthly intervals year‑round or at least within one month of the animal's first seasonal exposure to mosquitoes and continuing until at least 1 month after the dog's last seasonal exposure. If a dose is missed, give Simparica Trio immediately and resume monthly dosing. When replacing a monthly heartworm preventive product, Simparica Trio should be given within one month of the last dose of the former medication.
Flea Treatment and Prevention:
Treatment with Simparica Trio may begin at any time of the year. In areas where fleas are common year-round, monthly treatment with Simparica Trio can continue the entire year without interruption.
To minimize the likelihood of flea re-infestation, it is important to treat all dogs and cats within a household with an approved flea control product.
Tick Treatment and Control:
Treatment with Simparica Trio can begin at any time of the year (see Effectivenes).
Intestinal Nematode Treatment and Control:
For the treatment of roundworm (immature adult and adult Toxocara canis and adult Toxascaris leonina) and adult hookworm (Ancylostoma caninum and Uncinaria stenocephala) infections, Simparica Trio should be administered once as a single dose. Monthly use of Simparica Trio will control any subsequent infections.
How to Store Simparica Trio:
Store at or below 30°C (86°F). Keep away from children and pets.
Contraindications:
There are no known containdications for the use of Simparica Trio.
Precautions:
Sarolaner, one of the ingredients in Simparica Trio, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders.
Prior to administration of Simparica Trio, dogs should be tested for existing heartworm infections. Infected dogs should be treated with an adulticide to remove adult heartworms. Simparica Trio is not effective against adult D. immitis.
The safe use of Simparica Trio has not been evaluated in breeding, pregnant, or lactating dogs.
Just one Simparica Trio chewable delivered fully effective heartworm disease protection
In two well-controlled laboratory studies, a single oral dose of Simparica Trio was 100% effective in preventing the development of heartworm disease in dogs inoculated with infective larvae 30 days before treatment.

Simparica Trio was designed for balancing optimal effectiveness with safety to prevent heartworm disease


Possible Side Effects:
In a field safety and effectiveness study, Simparica Trio was administered to dogs for the prevention of heartworm disease. The study included a total of 410 dogs treated once monthly for 11 treatments (272 treated with Simparica Trio and 138 treated with an active control). Over the 330 day study period, all observations of potential adverse reactions were recorded. The most frequent reactions reported in the Simparica Trio group are presented in the following table.
| Dogs with Adverse Reactions | ||
|---|---|---|
| Clinical Sign | Simparica Trio n=272 | Active Controln=138 |
| Vomiting | 14.3% | 10.9% |
| Diarrhea | 13.2% | 8.0% |
| Lethargy | 8.5% | 6.5% |
| Anorexia | 5.1% | 5.8% |
| Polyuria | 3.7% | 3.6% |
| Hyperactivity | 2.2% | 0.7% |
| Polydipsia | 2.2% | 2.9% |
In a second field safety and effectiveness study, Simparica Trio was administered to 278 dogs with fleas. Adverse reactions in dogs treated with Simparica Trio included diarrhea.
In a third field safety and effectiveness study, Simparica Trio was administered to 120 dogs with roundworms. Adverse reactions in dogs treated with Simparica Trio included diarrhea and vomiting.
Simparica Trio Important Safety Information:
Use with caution in dogs with a history of seizures. Simparica Trio contains sarolaner, a member of the isoxazoline class, which has been associated with neurologic adverse reactions including tremors, ataxia, and seizures in dogs with or without a history of neurologic disorders. The safe use of Simparica Trio has not been evaluated in breeding, pregnant, or lactating dogs. The most frequently reported adverse reactions in clinical trials were vomiting and diarrhea. See full Prescribing Information, attached.
Effectiveness:
Heartworm Prevention
In two well controlled laboratory studies, a single oral dose of Simparica Trio was 100% effective in preventing the development of adult D. immitis in dogs inoculated with infective larvae 30 days before treatment.
In a well controlled US field study consisting of 246 dogs administered Simparica Trio and 119 administered an active control, no dogs treated with Simparica Trio tested positive for heartworm disease. All dogs treated with Simparica Trio were negative for D. immitis antigen and blood microfilariae at study completion on day 330.
Flea Treatment and Prevention
In a well controlled laboratory study, Simparica Trio began to kill fleas at 4 hours and demonstrated 100% effectiveness at 8 hours after initial administration. After weekly re-infestations, Simparica Trio reduced the number of live fleas by 97.8% within 12 hours of infestation for 28 days.
In a separate well controlled laboratory study, Simparica Trio demonstrated 100% effectiveness against adult fleas within 24 hours following treatment and maintained 99.7% effectiveness against weekly re-infestations for 35 days.
In a study to explore flea egg production and viability, Simparica Trio killed fleas before they could lay eggs for 35 days.
In a well-controlled 60-day US field study conducted in dogs with existing flea infestations of varying severity, the effectiveness of Simparica Trio against fleas on Day 30 and 60 visits was 99.0% and 99.7%, respectively, compared to baseline. Dogs with signs of flea allergy dermatitis showed improvement in erythema, papules, scaling, alopecia, dermatitis/pyodermatitis and pruritus as a direct result of eliminating fleas.
Tick Treatment and Control
In a well controlled laboratory study, Simparica Trio began to kill existing I. scapularis within 8 hours, Simparica Trio reduced the number of live ticks by 94.2% within 24 hours of infestation for 28 days.
In well controlled laboratory studies, Simparica Trio demonstrated 98.9% effectiveness against an existing infestation of Amblyomma maculatum, Ixodes scapularis, Rhipicephalus sanguineus, and Dermacentor variabilis 48 hours postadministration and maintained 90.4% effectiveness 48 hours after re-infestation for at least 28 days. Against Amblyomma americanum, Simparica Trio demonstrated 99.4% effectiveness 72 hours after treatment of existing infestations, and maintained 98.4% effectiveness 72 hours after re-infestation for at least 28 days.
Intestinal Nematode Treatment and Control
Elimination of roundworms (immature adult and adult Toxocara canis and adult Toxascaris leonina) and adult hookworms (Ancylostoma caninum and Uncinaria stenocephala) was demonstrated in well controlled laboratory studies.
In a 10-day multi-center field study, Simparica Trio was effective against Toxocara canis and reduced fecal egg counts 99.2%.
Animal Safety:
Margin of Safety
Simparica Trio was administered orally to 8 week old Beagle puppies at doses of 1, 3, and 5X the maximum labeled dose (2.4 mg/kg sarolaner, 48 ?/kg moxidectin, and 10 mg/kg pyrantel) at 28 day intervals for 7 treatments. Dogs in the control group received placebo. There were no clinically relevant, treatment related effects on clinical observations, body weights, food consumption, clinical pathology (hematology, coagulation, serum chemistry, and urinalysis), gross pathology, histopathology, or organ weights. During the end of study ophthalmic examination, the following change was found: one 1X dog had retinal dysplasia (OS folds).
Ivermectin-sensitive Collie Safety
Simparica Trio was administered orally once at 1, 3 and 5X the maximum labeled dose to Collies that had been pre-screened for avermectin sensitivity. Dogs in the control group received placebo. Clinical signs (ataxia, muscle fasciculations, mydriasis) associated with avermectin sensitivity were observed in the 5X group. All dogs were completely recovered by the third day of the study.
Heartworm-Positive Safety
Simparica Trio was administered orally at 1 and 3X the maximum labeled dose at 28 day intervals for 3 treatments to Beagle dogs with patent adult heartworm infections and circulating microfilariae. Dogs in the control group received placebo. Diarrhea occurred more commonly in the treated dogs and also more often in the 3X group compared with the 1X group. Two dogs (1 each in 1X and 3X) developed a fever less than 24 hours after the first dose. The fever may have been a transient reaction to a rapid microfilaria reduction. Both dogs recovered without treatment.
Field Safety
In three well-controlled field studies, Simparica Trio was used concurrently with other medications such as vaccines, antimicrobials, anthelmintics, antiprotozoals, steroidal and non-steroidal anti-inflammatory agents, anesthetic agents and analgesics. No adverse reactions were associated with the concurrent use of Simparica Trio and other medications.
FAQ
- Heartworm disease prevention - In two well-controlled laboratory studies, a single oral dose of Simparica Trio was 100% effective in preventing the development of heartworm disease in dogs.
- Tick treatment and control - In a well-controlled laboratory study, Simparica Trio began to kill deer ticks within 8 hours.
- Flea treatment and prevention - In a well-controlled laboratory study, Simparica Trio began to kill fleas at 4 hours and demonstrated 100% effectiveness at 8 hours after initial administration.
- Intestinal parasite treatment and control - In a 10-day multi-center field study, Simparica Trio was 99.2% effective against adult roundworm and reduction in fecal egg count.1,2† It also demonstrated >94% effective against hookworm, in studies.4
Dosage and Administration
- Can be given with or without food
- Available in 6-packs
- Store at or below 30°C (86°F)

Sarolaner is an acaricide and insecticide belonging to the isoxazoline group. Sarolaner inhibits the function of the neurotransmitter gamma aminobutyric acid (GABA) receptor and glutamate receptor and works at the neuromuscular junction in insects. This results in uncontrolled neuromuscular activity leading to death in insects or acarines.
Moxidectin is an endectocide in the macrocyclic lactone class. Moxidectin acts by interfering with the chloride channel-mediated neurotransmission in the parasite. This results in paralysis and death of the parasite.
Pyrantel pamoate is a nematocide belonging to the tetrahydropyrimidine class. Pyrantel acts as a depolarizing, neuromuscular-blocking agent in susceptible parasites, which causes paralysis and death or expulsion of the organism.
Safety demonstrated in:
- 8 week old puppies
- Ivermectin-sensitive dogs
- Heartworm-positive dogs*
- Dogs concurrently receiving other medications†
The three most common adverse events:
- Vomiting
- Diarrhea
- Lethargy
*Infected dogs should be treated with an adulticide to remove adult heartworms. Simparica Trio is not effective against adult D. immitis.
†While dogs received concurrent medications during field studies, no specific studies to demonstrate safety with concurrent use of other drugs were performed.
In clinical trials, Simparica Trio:
- Was 100% effective against heartworm disease in dogs after one dose
- Started killing fleas within 4 hours and achieved 100% efficacy 8 hours after treatment
- Was ≥98.9% effective against tick infestations in 48 hours‡
- Resulted in 99.2% efficacy for adult roundworm and reduction in fecal egg count, and >94% efficacy for hookworm1,2,4
‡Against Amblyomma americanum, Simparica Trio demonstrated 99.4% effectiveness 72 hours after treatment of existing infestations.
Sarolaner is an acaricide and insecticide belonging to the isoxazoline group. Sarolaner inhibits the function of the neurotransmitter gamma aminobutyric acid (GABA) receptor and glutamate receptor and works at the neuromuscular junction in insects. This results in uncontrolled neuromuscular activity leading to death in insects or acarines.
Moxidectin is an endectocide in the macrocyclic lactone class. Moxidectin acts by interfering with the chloride channel-mediated neurotransmission in the parasite. This results in paralysis and death of the parasite.
Pyrantel pamoate is a nematocide belonging to the tetrahydropyrimidine class. Pyrantel acts as a depolarizing, neuromuscular-blocking agent in susceptible parasites, which causes paralysis and death or expulsion of the organism.
Reviews
- Delivery was very slow (USPS) vs. your main competitor (UPS)
- Works great!
- No cons
- all in one
- Quick service
- Very expensive
- Always have to contact my vet for another prescription and
- Dweibe there in
- Person to pick out Nd have to mail it
- Excellent flea, tick & heartworm medicine
- Prices
- They
- works well
- cost


























![Simparica Trio for Dogs - 44.1-88 lbs (1 Chewable Tablet) - [Flea, Ticks & Heartworm] Video](https://img.youtube.com/vi/hnGOkPXO2Yc/0.jpg)
