SAVE 20% OFF 20% OFF Use Code PREZ20 *
FELYCIN-CA1 Sirolimus Delayed Release Tablets (0.4 mg per tablets)
- Description
- Ingredients
- Directions
- Reviews
Description
Felycin-CA1 (sirolimus delayed-release tablets) is a prescription cardiac medication formulated specifically for cats. It is indicated for the management of ventricular hypertrophy in cats diagnosed with subclinical hypertrophic cardiomyopathy (HCM). By utilizing a delayed-release formulation, Felycin-CA1 allows controlled absorption of sirolimus to support long-term cardiac health while maintaining consistent therapeutic exposure.
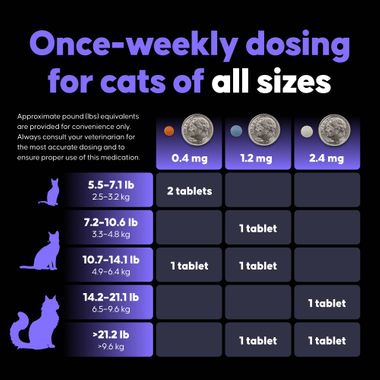
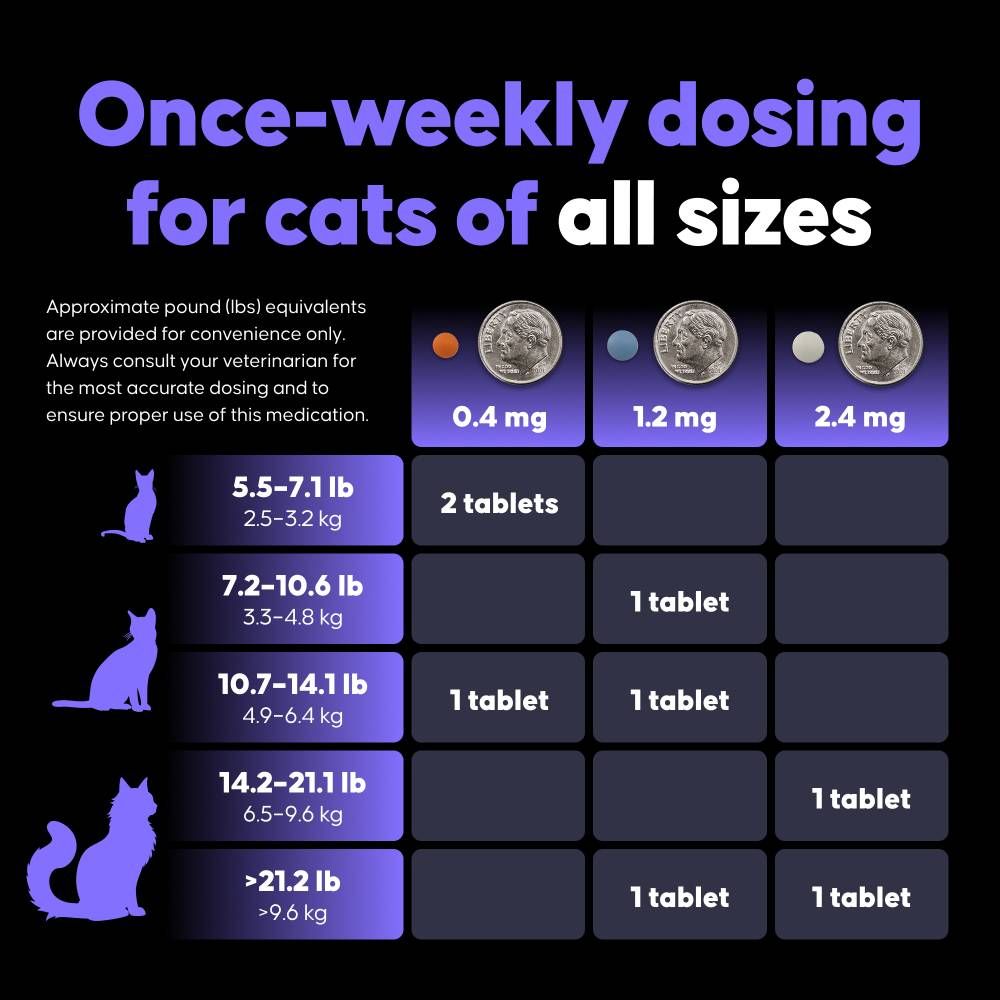
This oral medication is administered once weekly and should be swallowed whole with food to ensure proper absorption. Felycin-CA1 is conditionally approved by the FDA pending full demonstration of effectiveness and is available by prescription only through a licensed veterinarian.
- Helps manage ventricular hypertrophy associated with subclinical hypertrophic cardiomyopathy (HCM) in cats
- Contains sirolimus in a delayed-release tablet for controlled absorption
- Once-weekly oral dosing supports long-term treatment compliance
- Designed specifically for feline cardiac use
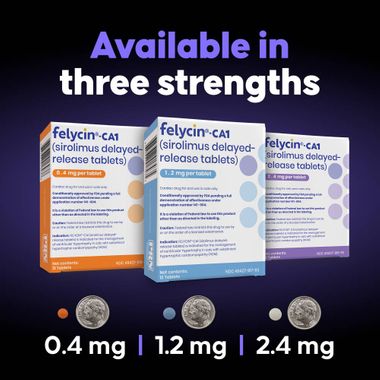
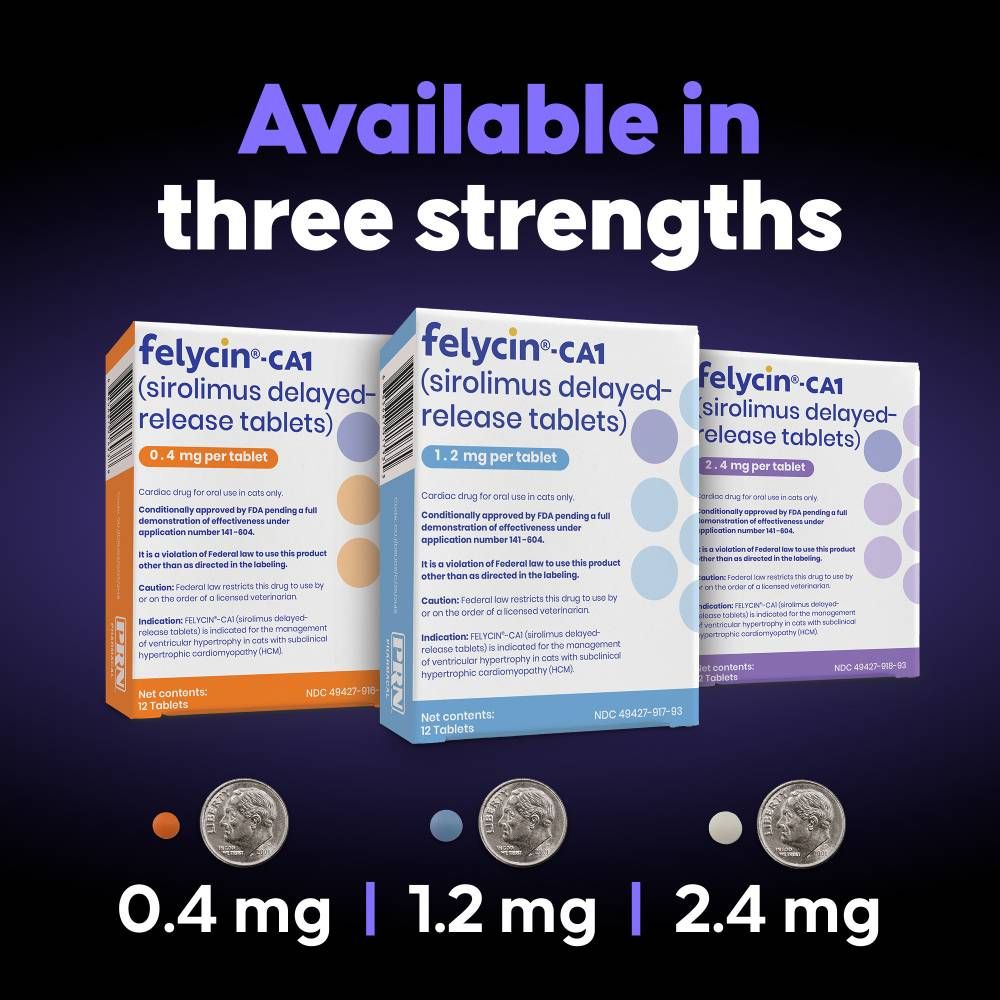
- Available in multiple strengths to support accurate weight-based dosing
- Conditionally approved by the FDA for veterinary use
Ingredients
| Type | Details |
|---|---|
| Active Ingredient | Sirolimus |
| Inactive Ingredients | Inactive ingredients are not listed on the outer carton. Please refer to the full package insert for complete excipient information. |
Directions
Directions for Use
| Dosage | 0.3 mg/kg orally once weekly, or as directed by a licensed veterinarian. |
| Administration | Tablets must be swallowed whole. Do not chew or crush. |
| Feeding | Administer in conjunction with a meal to support proper absorption. |
Warnings & Precautions
| Veterinary Use | For veterinary use only. Federal law restricts this drug to use by or on the order of a licensed veterinarian. |
| Human Safety | Not for use in humans. Keep out of reach of children. In case of accidental human ingestion, seek medical attention immediately and provide the product label or package insert. |
| Handling Precautions | Pregnant or breastfeeding women should avoid contact with this medication. Individuals with known hypersensitivity to sirolimus should use caution. |
| Exposure Risk | Avoid direct contact with vomit, saliva, or tablet remnants. Wear gloves when cleaning and wash hands thoroughly afterward. Dispose of any tablets not swallowed immediately. |
| Animal Safety | Keep secured and out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose. |
Storage
| Packaging | Store tablets in the original packaging. Only remove tablets from the blister at the time of dosing. |
| Conditions | Store at controlled room temperature. Protect from moisture and excessive heat. |